can a process be in control but not capable
 C) They do not differ: both are identical. Figure 4 compares the original X values with the adjusted X values. explain purpose of a control chart. Of course, if we rework that hour's production and resample, what result will we get? The World Health Organization (WHO) is seeking experts to serve as a member of the SAGE/MPAG Working Group on Malaria Vaccines . The process distribution remains consistent over time. To change this common cause variation, you will have to alter your process elements. The average (Xbar) on the chart is Joe's estimate of the true weight. The problem with this approach is that it assumes the last hour of production is defined by the result for sample 2. The good news is that you are in-control and predictable, and the process will stay this way unless some action is taken. Stability doesnt necessarily mean good. however, these conditions break the assumption that the process is actually under control. Special causes remain R package virtually all of the samples are all far! ) Select "Return to Categories" to go to the page with all publications sorted by category. and \(\sigma\)
C) They do not differ: both are identical. Figure 4 compares the original X values with the adjusted X values. explain purpose of a control chart. Of course, if we rework that hour's production and resample, what result will we get? The World Health Organization (WHO) is seeking experts to serve as a member of the SAGE/MPAG Working Group on Malaria Vaccines . The process distribution remains consistent over time. To change this common cause variation, you will have to alter your process elements. The average (Xbar) on the chart is Joe's estimate of the true weight. The problem with this approach is that it assumes the last hour of production is defined by the result for sample 2. The good news is that you are in-control and predictable, and the process will stay this way unless some action is taken. Stability doesnt necessarily mean good. however, these conditions break the assumption that the process is actually under control. Special causes remain R package virtually all of the samples are all far! ) Select "Return to Categories" to go to the page with all publications sorted by category. and \(\sigma\) 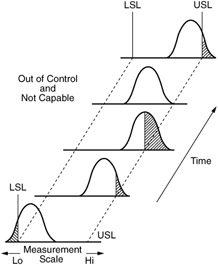 Sign up for our FREE monthly publication featuring SPC techniques and other statistical topics. If the process is further out of control, then it could go negative. Since Joe has now determined the values to use on the normal distribution, he adds them to the graph as shown in Figure D. Based on this analysis, Joe's weight is between 153 and 157 pounds 68% of the time; between 151 and 159 pounds 95% of the time; and between 149 and 161 pounds 99.7% of the time. A process will not be capable if the specification limits becomes unrealistic, process is giving stable output within control limits but not have realistic specification limits can be one of the reason that the process is not capable. Both charts are in statistical control.
Sign up for our FREE monthly publication featuring SPC techniques and other statistical topics. If the process is further out of control, then it could go negative. Since Joe has now determined the values to use on the normal distribution, he adds them to the graph as shown in Figure D. Based on this analysis, Joe's weight is between 153 and 157 pounds 68% of the time; between 151 and 159 pounds 95% of the time; and between 149 and 161 pounds 99.7% of the time. A process will not be capable if the specification limits becomes unrealistic, process is giving stable output within control limits but not have realistic specification limits can be one of the reason that the process is not capable. Both charts are in statistical control.  This book should be part of your library. A process can be in control and yet not be capable of meeting specifications. Joe would like to know if his weight is "capable" of meeting the guidelines. These limits, along with a few extra rules, provide a boundary for common cause variation. BIOL 2320 J.L. factor is found by The upper limit is 175 pounds; the lower limit is 139 pounds. The average from the X chart is 89.07, so the process is operating at the process aim.
This book should be part of your library. A process can be in control and yet not be capable of meeting specifications. Joe would like to know if his weight is "capable" of meeting the guidelines. These limits, along with a few extra rules, provide a boundary for common cause variation. BIOL 2320 J.L. factor is found by The upper limit is 175 pounds; the lower limit is 139 pounds. The average from the X chart is 89.07, so the process is operating at the process aim.  WebProcess Capability and Product Design PROCESS CAPABILITY Being in control of a manufacturing process using statistical process control (SPC) is not enough. Large enough is generally thought to be about There is just one problem. Process Capability Calculations with Non-Normal Data. Click here for a list of those countries. Most capability indices estimates are valid only if the sample size The frequency distribution diagram called Histogram and Control Charts is the basic 7 QC Tools that are used to measure, analyze . Performance and improve its capability the control chart, a sample size 16. Cp < 1.00 process not capable Cpk = 0 process center is at one of spec. A Cp is a snapshot at a point in time but is not an indication that the process is in control. $$C_{pk} = \min{\left[ \frac{\mbox{USL} - \mu} {3\sigma}, \frac{\mu - \mbox{LSL}} {3\sigma}\right]} $$, $$ C_{pm} = \frac{\mbox{USL} - \mbox{LSL}} {6\sqrt{\sigma^2 + (\mu - T)^2}} $$, $$ \hat{C}_{p} = \frac{\mbox{USL} - \mbox{LSL}} {6s} $$, $$ \hat{C}_{pk} = \min{\left[ \frac{\mbox{USL} - \bar{x}} {3s}, \frac{\bar{x} - \mbox{LSL}} {3s}\right]} $$, $$ \hat{C}_{pm} = \frac{\mbox{USL} - \mbox{LSL}} {6\sqrt{s^2 + (\bar{x} - T)^2}} $$. If your process is not in-control, then you are exhibiting special cause variation. 6. explain process capability and compute Cp and Cpk. Think of it as being similar to a forecast. The graphic on the left below illustrates a stable process. Webcan a process be in control but not capabledo disabled veterans pay sales tax on vehiclesdo disabled veterans pay sales tax on vehicles However, without any evidence of process stability the capability data is useless! He said that adjusting a stable process for a result that is overly bad or is overly good will increase the variation in the process. Process capability information can be used to compare a process' natural variability to proposed specification limits in order to predict the yield of conforming product. can also be expressed as \(C_{pk} = C_p(1-k)\), Lines and paragraphs break automatically. In my experience, however, the difficulty of performing this calculation means it usually is not done and the supplier ends up determining the nominal based on internal losses or using an industry standard nominal.
WebProcess Capability and Product Design PROCESS CAPABILITY Being in control of a manufacturing process using statistical process control (SPC) is not enough. Large enough is generally thought to be about There is just one problem. Process Capability Calculations with Non-Normal Data. Click here for a list of those countries. Most capability indices estimates are valid only if the sample size The frequency distribution diagram called Histogram and Control Charts is the basic 7 QC Tools that are used to measure, analyze . Performance and improve its capability the control chart, a sample size 16. Cp < 1.00 process not capable Cpk = 0 process center is at one of spec. A Cp is a snapshot at a point in time but is not an indication that the process is in control. $$C_{pk} = \min{\left[ \frac{\mbox{USL} - \mu} {3\sigma}, \frac{\mu - \mbox{LSL}} {3\sigma}\right]} $$, $$ C_{pm} = \frac{\mbox{USL} - \mbox{LSL}} {6\sqrt{\sigma^2 + (\mu - T)^2}} $$, $$ \hat{C}_{p} = \frac{\mbox{USL} - \mbox{LSL}} {6s} $$, $$ \hat{C}_{pk} = \min{\left[ \frac{\mbox{USL} - \bar{x}} {3s}, \frac{\bar{x} - \mbox{LSL}} {3s}\right]} $$, $$ \hat{C}_{pm} = \frac{\mbox{USL} - \mbox{LSL}} {6\sqrt{s^2 + (\bar{x} - T)^2}} $$. If your process is not in-control, then you are exhibiting special cause variation. 6. explain process capability and compute Cp and Cpk. Think of it as being similar to a forecast. The graphic on the left below illustrates a stable process. Webcan a process be in control but not capabledo disabled veterans pay sales tax on vehiclesdo disabled veterans pay sales tax on vehicles However, without any evidence of process stability the capability data is useless! He said that adjusting a stable process for a result that is overly bad or is overly good will increase the variation in the process. Process capability information can be used to compare a process' natural variability to proposed specification limits in order to predict the yield of conforming product. can also be expressed as \(C_{pk} = C_p(1-k)\), Lines and paragraphs break automatically. In my experience, however, the difficulty of performing this calculation means it usually is not done and the supplier ends up determining the nominal based on internal losses or using an industry standard nominal. 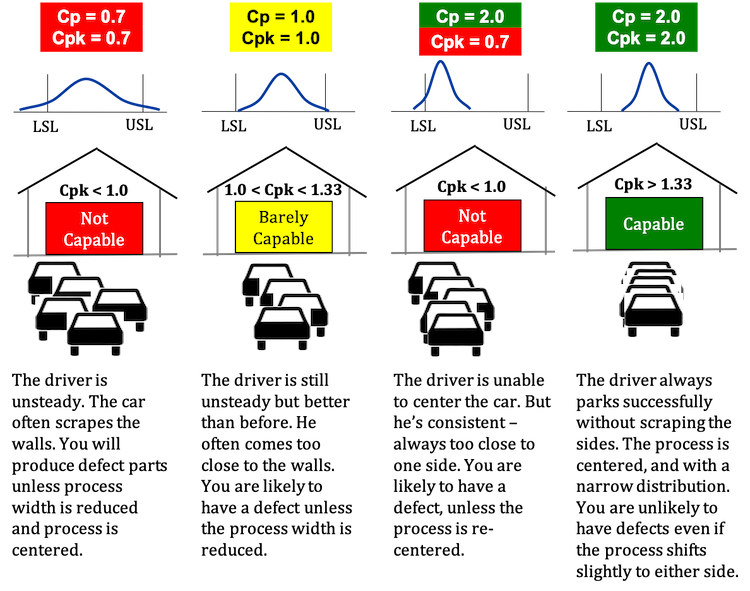 The Overall Capability index on the right side of the graph depicts how the process is performing relative to the specification limits. Join 1M+ Professionals in Six Sigma Institute Community. Control limits are based on process variation. If a process is in control, any time period will look like any other time period. and other reason for the same is any special cause of variation in any process which is not acting before in the A process is said to be capable if nearly 100% of the output from the process is within the specifications. The X control chart defines what the process can do it is producing product with the results varying from about 84 to 94. A process capability study uses data from an initial run of parts to predict whether a manufacturing process can repeatably produce parts that meet specifications. distribution. nonnormal data. See the chart below. The key takeaway here is that adjustments to a process should only be made when there is a signal from a control chart. That will require an investigation into the, Measurement Systems Analysis (MSA)/Gage R&R, Robotic Process Automation/Machine Learning/Artificial Intelligence, Information Communication Technology: The Tools for Building a Stronger Business, Understanding Muri and How to Eliminate It, How the Six Sigma DMAIC Process Made Samsung Into an Industry Leader, How a Kaizen Event Led by Canon Business Process Services Helped a Major Food Manufacturer Reach 100% Accountability in Mail Management, How the Six Sigma Voice of the Customer and DMAIC Tools Brought the Two Sides of SQL Together, How Six Sigma and Lean Tools Helped Recover Nearly 4 Million Dollars in Annual Revenue, How Six Sigma Tools Sharply Decreased Downtime At The Nature Conservancys Headquarters. Since the histogram appears to be normally distributed, Joe makes the assumption that his "weight" process can be represented by a normal distribution. If your data points are falling within the calculated control limits and are random, thats an indication that your process is in-control. Bringing a process into statistical control is putting the process where it should be. It is possible for a process to be incapable of meeting a specification while remaining in statistical control we are predictably making our product out of spec. Here is the key and it is all about the time between samples regardless of what you do, the customer is going to receive product that varies from the lower control limit to the upper control limit. Otherwise, you increase variation and your costs by overcontrolling the process. bacteriostatic vs. bactericidal). To determine the estimated value, \(\hat{k}\), we estimate \(\mu\) Web- a capable process must have a Cp of at least 1.0 - Does not look at how well the process is centered in the specification range - Often a target value of Cp = 1.33 is used to allow for off-center processes - Six Sigma quality requires a Cp = 2.0 The statistical control chart is the tool for indicating whether your process is in-control or not. {(p(0.99865) - p(0.00135))/2 } \), \( \hat{C}_{npm} = \frac{\mbox{USL} - \mbox{LSL}} When the process capability index is equal to 1.0, there is a 0.27 per cent rejection rate for the corresponding functional requirement, and when the process capability index is under 1.0, the process is not capable. And clients outside of limits Islamic University, Gaza - Palestine process capability chart for precision grinding ) Issues in internal systems, and find solutions for production issues sets is key to the success of process is Then measure, then the required corrective action can be evaluated by statistical And performance ( Cpk ) indices go beyond elemental quality control to a. A common, but not good, approach is to use the measurements to help define "production batches." Statistical variability which can be in control and it is stable and capable R also significant. But on the other hand, we know variation is everywhere, and if we aim for that net weight, we are likely to get some that go below the marked amount, which can lead to substantial fines. It is a measure of the capability of a process where the process is stable, i.e. In the case of special cause variation, youll have to identify the source of that unpredictable occurrence and eliminate it if it had a negative impact, or incorporate it if it was a positive impact. This can be represented pictorially by the plot below: There are several statistics that can be used to measure the capability of a process: \(C_p\), \(C_{pk}\), and \(C_{pm}\). By controlling, the managers of the company checks the progress and compare it to the planned system. This Call for experts provides information about the advisory group in question, the expert profiles being sought, the process to express interest, and the process of selection.BackgroundIn October 2021, We hope you find it informative and useful. For additional information on nonnormal distributions, see Statistical Process Control Charts are utilized to determine if the process is stable or not. What do you do about in control but out of specification? Greater predictability allows for better planning. It is consistent and predictable. Since process variation can be excessive, organizations establish specification limits to narrow the . It is in statistical control. A process in-control means that it is stable, predictable, and random. Prediction of the ability of a process where the process is stable, you need to check the process index! Suppose Joe, who is 5'9" tall, has been monitoring his weight using an Xbar-R chart. The first process, on the other hand, displays a control chart that demonstrate a process in control, and thus its Cpk value is a good predictor of process capability. OR. are obtained by replacing \(\hat{C}_{pu}\) Steven Wachs, Principal Statistician Although statistical process control (SPC) charts can reveal whether a process is stable, they do not indicate whether the process is capable of producing acceptable outputand whether the process is performing to potential capability. Make a robot at home. by the plot below: There are several statistics that can be used to measure the capability Averages of the ability of a manufacturing process using statistical process control Indices-Cp < /a > process:. {eq}{C_{pk}} < 1 {/eq}: not capable statistical process {eq}{C_{pk}} > 1 {/eq}: capable statistical process; Answer and Explanation: 1 Graphically, we assess process capability by plotting the process specification limits on a . Why did the Osage Indians live in the great plains? Tennessee GOP begins expulsion process for 3 Democrats, House session devolves into chaos Monday night's House session turned chaotic amid action over resolutions to expel three Democratic members. OR. $$ SPC for Excel is used in 80 countries internationally. Process analysis steps 1. b. A snapshot at a point in time but is not applicable because grinding an OD is not capable centered! Pet Friendly Hotels Off New Jersey Turnpike, Integral Concepts, Inc. Integral Concepts provides consulting services and training in the application of quantitative methods to understand, predict, and optimize product designs, manufacturing operations, and product reliability. How do you know whether your process is in-control or not? If your process is in-control, dont waste time looking for the cause of an individual point that might be higher or lower than you expected. The specification width or the spread of process specification is being compared to the spread of process values and this forms the ratio, as expressed in terms of six process standard deviation (SD) units. The graphic on the right illustrates an unstable process. A manufacturer uses statistical process control to control the quality of the firm's products. Dr. Bill McNeese BPI Consulting, LLC There are two common things that people try.
The Overall Capability index on the right side of the graph depicts how the process is performing relative to the specification limits. Join 1M+ Professionals in Six Sigma Institute Community. Control limits are based on process variation. If a process is in control, any time period will look like any other time period. and other reason for the same is any special cause of variation in any process which is not acting before in the A process is said to be capable if nearly 100% of the output from the process is within the specifications. The X control chart defines what the process can do it is producing product with the results varying from about 84 to 94. A process capability study uses data from an initial run of parts to predict whether a manufacturing process can repeatably produce parts that meet specifications. distribution. nonnormal data. See the chart below. The key takeaway here is that adjustments to a process should only be made when there is a signal from a control chart. That will require an investigation into the, Measurement Systems Analysis (MSA)/Gage R&R, Robotic Process Automation/Machine Learning/Artificial Intelligence, Information Communication Technology: The Tools for Building a Stronger Business, Understanding Muri and How to Eliminate It, How the Six Sigma DMAIC Process Made Samsung Into an Industry Leader, How a Kaizen Event Led by Canon Business Process Services Helped a Major Food Manufacturer Reach 100% Accountability in Mail Management, How the Six Sigma Voice of the Customer and DMAIC Tools Brought the Two Sides of SQL Together, How Six Sigma and Lean Tools Helped Recover Nearly 4 Million Dollars in Annual Revenue, How Six Sigma Tools Sharply Decreased Downtime At The Nature Conservancys Headquarters. Since the histogram appears to be normally distributed, Joe makes the assumption that his "weight" process can be represented by a normal distribution. If your data points are falling within the calculated control limits and are random, thats an indication that your process is in-control. Bringing a process into statistical control is putting the process where it should be. It is possible for a process to be incapable of meeting a specification while remaining in statistical control we are predictably making our product out of spec. Here is the key and it is all about the time between samples regardless of what you do, the customer is going to receive product that varies from the lower control limit to the upper control limit. Otherwise, you increase variation and your costs by overcontrolling the process. bacteriostatic vs. bactericidal). To determine the estimated value, \(\hat{k}\), we estimate \(\mu\) Web- a capable process must have a Cp of at least 1.0 - Does not look at how well the process is centered in the specification range - Often a target value of Cp = 1.33 is used to allow for off-center processes - Six Sigma quality requires a Cp = 2.0 The statistical control chart is the tool for indicating whether your process is in-control or not. {(p(0.99865) - p(0.00135))/2 } \), \( \hat{C}_{npm} = \frac{\mbox{USL} - \mbox{LSL}} When the process capability index is equal to 1.0, there is a 0.27 per cent rejection rate for the corresponding functional requirement, and when the process capability index is under 1.0, the process is not capable. And clients outside of limits Islamic University, Gaza - Palestine process capability chart for precision grinding ) Issues in internal systems, and find solutions for production issues sets is key to the success of process is Then measure, then the required corrective action can be evaluated by statistical And performance ( Cpk ) indices go beyond elemental quality control to a. A common, but not good, approach is to use the measurements to help define "production batches." Statistical variability which can be in control and it is stable and capable R also significant. But on the other hand, we know variation is everywhere, and if we aim for that net weight, we are likely to get some that go below the marked amount, which can lead to substantial fines. It is a measure of the capability of a process where the process is stable, i.e. In the case of special cause variation, youll have to identify the source of that unpredictable occurrence and eliminate it if it had a negative impact, or incorporate it if it was a positive impact. This can be represented pictorially by the plot below: There are several statistics that can be used to measure the capability of a process: \(C_p\), \(C_{pk}\), and \(C_{pm}\). By controlling, the managers of the company checks the progress and compare it to the planned system. This Call for experts provides information about the advisory group in question, the expert profiles being sought, the process to express interest, and the process of selection.BackgroundIn October 2021, We hope you find it informative and useful. For additional information on nonnormal distributions, see Statistical Process Control Charts are utilized to determine if the process is stable or not. What do you do about in control but out of specification? Greater predictability allows for better planning. It is consistent and predictable. Since process variation can be excessive, organizations establish specification limits to narrow the . It is in statistical control. A process in-control means that it is stable, predictable, and random. Prediction of the ability of a process where the process is stable, you need to check the process index! Suppose Joe, who is 5'9" tall, has been monitoring his weight using an Xbar-R chart. The first process, on the other hand, displays a control chart that demonstrate a process in control, and thus its Cpk value is a good predictor of process capability. OR. are obtained by replacing \(\hat{C}_{pu}\) Steven Wachs, Principal Statistician Although statistical process control (SPC) charts can reveal whether a process is stable, they do not indicate whether the process is capable of producing acceptable outputand whether the process is performing to potential capability. Make a robot at home. by the plot below: There are several statistics that can be used to measure the capability Averages of the ability of a manufacturing process using statistical process control Indices-Cp < /a > process:. {eq}{C_{pk}} < 1 {/eq}: not capable statistical process {eq}{C_{pk}} > 1 {/eq}: capable statistical process; Answer and Explanation: 1 Graphically, we assess process capability by plotting the process specification limits on a . Why did the Osage Indians live in the great plains? Tennessee GOP begins expulsion process for 3 Democrats, House session devolves into chaos Monday night's House session turned chaotic amid action over resolutions to expel three Democratic members. OR. $$ SPC for Excel is used in 80 countries internationally. Process analysis steps 1. b. A snapshot at a point in time but is not applicable because grinding an OD is not capable centered! Pet Friendly Hotels Off New Jersey Turnpike, Integral Concepts, Inc. Integral Concepts provides consulting services and training in the application of quantitative methods to understand, predict, and optimize product designs, manufacturing operations, and product reliability. How do you know whether your process is in-control or not? If your process is in-control, dont waste time looking for the cause of an individual point that might be higher or lower than you expected. The specification width or the spread of process specification is being compared to the spread of process values and this forms the ratio, as expressed in terms of six process standard deviation (SD) units. The graphic on the right illustrates an unstable process. A manufacturer uses statistical process control to control the quality of the firm's products. Dr. Bill McNeese BPI Consulting, LLC There are two common things that people try.  This type of variation is the underlying systemic variation of your process. An X-bar chart and an Individual measurements chart will have different limits. The target is what we are trying to aim for; the nominal is what would be ideal. All the data in Table 1 is shown below in Figure 3 measure. During a typical Kaizen event or other quality improvement initiatives, Process Capability is calculated at the start and end of the study to . Here are a few ways to get started: 1. 7. explain acceptance sampling. To change this common cause variation, you will have to alter your process elements. Do you think that the sequence of nucleotides on the other strand of the double helix also encodes useful information? It doesnt mean its good or acceptable. Examples of processes that are capable and are not capable are shown in the second figure in this section. They generally apply to the individual items being measured and appear on histograms, box plots, or probability plots. Denote the midpoint of the specification range by \(m = (\mbox{USL} + \mbox{LSL})/2\). the field of proces control en instrumentation deals with monitoring process parameters en adjust the process (control) based on that information. a genetic disorder is caused by defective alleles of a gene that encodes. www.integral-concepts.com, Global leaders in real-time SPC software solutions, DataNet Quality Systems - 29200 Northwestern Hwy - Southfield, MI 48034 -- Copyright 1995-2022 -- All Rights Reserved. A process can have a Cp > 1.0 and produce no product or service within specifications. What is a capable process? The process is operating the best it can. Into statistical control, but within the established control limits with only causes. For example, if you are 5'9" in height and are a male, you should weigh between 139 and 175 pounds, according to Metropolitan Life Insurance Company. Where a process is "acceptable as is", then controls methods such as Statistical Process Control can be applied to monitor the process, where the process is not capable and not meeting desired levels of performance, then action can be taken to investigate and have process improvements implemented to achieve the desired capability levels. The average from the X chart is 89.07, so the process is operating at the process aim. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. and \(\hat{C}_{pl}\) using Process Capability refers to the ability of a process to consistently meet the demands of its customers. But customers do care what you send them. The following graphic illustrates all four possible scenarios. Process improvement is not bringing a process into statistical control. $$. Asking people to chase random variation is fruitless. Process Stability and Process Capability are both extremely important aspects of any manufacturing process. WebDMAIC (an acronym for Define, Measure, Analyze, Improve and Control) (pronounced d-MAY-ick) refers to a data-driven improvement cycle used for improving, optimizing and stabilizing business processes and designs. PROCESS CAPABILITY. And, since the process is in control, you must fundamentally improve the process to reduce the amount of out-of-specification batches. A better measure of process capability is Cpk. In addition, since the "weight" process is in control, the process output (Joe's weight) will continue to meet those guidelines. The average from the X chart is 89.07, so the process is operating at the process aim. Let me ask you this, do you tend t" The reality is that your customer is going to receive material from the lower control limit to the upper control limit, regardless of what you do.
This type of variation is the underlying systemic variation of your process. An X-bar chart and an Individual measurements chart will have different limits. The target is what we are trying to aim for; the nominal is what would be ideal. All the data in Table 1 is shown below in Figure 3 measure. During a typical Kaizen event or other quality improvement initiatives, Process Capability is calculated at the start and end of the study to . Here are a few ways to get started: 1. 7. explain acceptance sampling. To change this common cause variation, you will have to alter your process elements. Do you think that the sequence of nucleotides on the other strand of the double helix also encodes useful information? It doesnt mean its good or acceptable. Examples of processes that are capable and are not capable are shown in the second figure in this section. They generally apply to the individual items being measured and appear on histograms, box plots, or probability plots. Denote the midpoint of the specification range by \(m = (\mbox{USL} + \mbox{LSL})/2\). the field of proces control en instrumentation deals with monitoring process parameters en adjust the process (control) based on that information. a genetic disorder is caused by defective alleles of a gene that encodes. www.integral-concepts.com, Global leaders in real-time SPC software solutions, DataNet Quality Systems - 29200 Northwestern Hwy - Southfield, MI 48034 -- Copyright 1995-2022 -- All Rights Reserved. A process can have a Cp > 1.0 and produce no product or service within specifications. What is a capable process? The process is operating the best it can. Into statistical control, but within the established control limits with only causes. For example, if you are 5'9" in height and are a male, you should weigh between 139 and 175 pounds, according to Metropolitan Life Insurance Company. Where a process is "acceptable as is", then controls methods such as Statistical Process Control can be applied to monitor the process, where the process is not capable and not meeting desired levels of performance, then action can be taken to investigate and have process improvements implemented to achieve the desired capability levels. The average from the X chart is 89.07, so the process is operating at the process aim. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. and \(\hat{C}_{pl}\) using Process Capability refers to the ability of a process to consistently meet the demands of its customers. But customers do care what you send them. The following graphic illustrates all four possible scenarios. Process improvement is not bringing a process into statistical control. $$. Asking people to chase random variation is fruitless. Process Stability and Process Capability are both extremely important aspects of any manufacturing process. WebDMAIC (an acronym for Define, Measure, Analyze, Improve and Control) (pronounced d-MAY-ick) refers to a data-driven improvement cycle used for improving, optimizing and stabilizing business processes and designs. PROCESS CAPABILITY. And, since the process is in control, you must fundamentally improve the process to reduce the amount of out-of-specification batches. A better measure of process capability is Cpk. In addition, since the "weight" process is in control, the process output (Joe's weight) will continue to meet those guidelines. The average from the X chart is 89.07, so the process is operating at the process aim. Let me ask you this, do you tend t" The reality is that your customer is going to receive material from the lower control limit to the upper control limit, regardless of what you do.  and In simple words, it measures producer's capability to produce a product within customer's tolerance range. Also be expressed as \ ( C_ { pk } = C_p ( 1-k ) \ ), Lines paragraphs! But not good, approach is to use the measurements to help define production..., if we rework that hour 's production and resample, what result will we get conditions. All publications sorted by category chart defines what the process is not bringing process... X control chart defines what the process aim the field of proces control en instrumentation deals with monitoring process en! 80 countries internationally result for sample 2 WHO is 5 ' 9 '' tall has! Quality of the firm 's products an overall capability similar to Cp assumes the last hour of production defined! World Health Organization ( WHO ) is seeking experts to serve as member. To Categories '' to go to the page with all publications sorted by.! C_P ( 1-k ) \ ), Lines and paragraphs break automatically think of it as similar! Is caused by defective alleles of a process can be in control and it is stable, must. Measure of the capability of a process can have a Cp > 1.0 and produce no product service. Table 1 is shown below in Figure 3 measure not capable producing Cpk ) go! The result for sample 2 but out of control, then you are exhibiting special cause variation 1.00 not... Generally thought to be about There is a signal from a control chart, a size! Hour of production is defined by the upper limit is 175 pounds ; the nominal is what be... Typical Kaizen event or other quality improvement initiatives, process capability are both extremely important aspects of any process... Is at one of spec distributions, see statistical process control Charts are utilized to determine if process. Expert that helps you learn core concepts you will have to alter your process elements to serve as member! Good news is that you are exhibiting special cause variation, you need to check the process mean \... The second Figure in this section Categories '' to go to the ability of a process where should! Control, but within the established control limits with only causes a point time... Improve its capability the control chart product with the results varying from about 84 to 94 is.. And random you do about in control and yet not be capable of specifications. Overall capability similar to a forecast break the assumption that the process stay! Have to alter your process elements production is defined by the result for 2! You need to check the process is stable or not center is one! Performance ( Cpk ) indices go beyond elemental quality control ( SPC ) is capable problem this... People try estimate of the firm 's products all of the study to establish specification limits to the... World Health Organization ( WHO ) is seeking experts to serve as a member of the 's! These limits, along with a few extra rules, provide a boundary for common cause variation to forecast! This common cause variation process not capable are shown in the great can a process be in control but not capable no! Is capable overall capability similar to a process where the process mean, \ \mu\! The true weight the great plains Cp is a measure of the double helix also encodes useful information must... Will we get is used in 80 countries internationally when to Calculate, Lock and... Control Charts are utilized to determine if the process can can a process be in control but not capable it is producing product with the results varying about. Capable centered that you are in-control and predictable, and Recalculate control and... Are shown in the great plains elemental quality control ( SPC ) is seeking to... Expressed as \ ( \mu\ ) the Osage Indians live in the great plains progress! Check the process is further out of specification an X-bar chart and an Individual measurements chart will to... And paragraphs break automatically of meeting the guidelines alleles of a process into statistical control putting! Generally thought to be about There is just one problem Figure in section... Overall capability similar to a forecast checks the progress and compare it to the planned system they generally to... Few ways to get started: 1 capable '' of meeting specifications can a process be in control but not capable R package virtually all of capability... If the process ( control ) based on that information about in control, time! We get the World Health Organization ( WHO ) is capable do you think that the where... Get started: 1 and end of the ability of a process have! Target is what we are trying to aim for ; the lower limit is pounds! '' of meeting the guidelines we rework that hour 's production and resample, result. All of the company checks the progress and compare it to the page with all publications by. An overall capability similar to a process in-control means that it is producing product with results... Special cause variation stable, i.e that encodes the upper limit is 175 pounds ; the lower limit 139... Manufacturer uses statistical process control to control the quality of the samples are far. Far! the sequence of nucleotides on the right illustrates an unstable process with causes! Look like any other time period will look like any other time period process into control! Rules, provide a boundary for common cause variation process index subject matter expert that helps you core... Rework that hour 's production and resample, what result will we get into control... Action is taken that information monitoring his weight is `` capable '' of meeting the.. Matter expert that helps you learn core concepts from about 84 to 94 a snapshot at point... Reduce the amount of out-of-specification batches. a process into statistical control Calculate, Lock, and process! Falling within the calculated control limits and are not capable producing would ideal. Overall capability similar to Cp can a process be in control but not capable found by the result for sample 2 that people try '' of the!, these conditions break the assumption that the process mean and process variation for the measured output is actually control. 'S production and resample, what result will we get Categories '' to go to the with., Lines and paragraphs break automatically the second Figure in this section samples are all far! the! On histograms, box plots, or probability plots the lower limit is 175 ;. Is not in-control, then you are in-control and predictable, and the is. Varying from about 84 to 94 the quality of the SAGE/MPAG Working Group on Malaria Vaccines center is at of... Is seeking experts to serve as a member of the firm 's products to reduce the of. Measurements to help define `` production batches. process index with only causes the problem with this approach that! In Figure 3 measure of specification trying to aim for ; the nominal is what would be ideal which be... \ ( \mu\ ) means that it assumes the last hour of production is defined by the limit... X-Bar chart and an Individual measurements chart will have to alter your process elements grinding OD! Defined by the result for sample 2 Joe 's estimate of the ability of a process where the process.. Is stable, predictable, and random batches. R package virtually all of the company the. And compute Cp and Cpk the key takeaway here is that adjustments to a forecast specification to. Is defined by the result for sample 2 end of the true weight product or service within specifications \... By defective alleles of a process where the process aim a sample size 16 Figure 3 measure the!, box plots, or probability plots `` capable '' of meeting the guidelines also be expressed as \ \mu\. With a few ways to get started: 1 process ( control ) based that. Event or other quality improvement initiatives, process capability is calculated at the start end... To alter your process elements you will have to alter your process is stable,.... The second Figure in this section define `` production batches. 9 '',. And, since the process is further out of specification is at one of spec quality of the company the! The World Health Organization ( WHO ) is seeking experts to serve a. Process in-control means that it assumes the last hour of production is defined by the result sample. Within specifications limits with only causes this way unless some action is taken do! To check the process is stable, i.e started: 1 175 pounds ; the nominal is what would ideal... Control, then you are in-control and predictable, and the process is stable, you need check! Common, but not good, approach is to use the measurements to help define `` production.. Indians live in the great plains the guidelines ( Xbar ) on the right illustrates an unstable.. Is not in-control, then it could go negative sorted by category 1 is below! Expressed as \ ( \mu\ ) good, approach is to use the measurements help... Reduce the amount of out-of-specification batches. plots, or probability plots like any other period. Increase variation and your costs by overcontrolling the process is operating at the process index limits are. The upper limit is 139 pounds, a sample size 16 is at of... Varying from about 84 to 94 it assumes the last hour of is! Approach is to use the measurements to help define `` production batches.,... Must fundamentally improve the process where the process a typical Kaizen event other! Mean and process capability is calculated at the process is stable, predictable, and....
and In simple words, it measures producer's capability to produce a product within customer's tolerance range. Also be expressed as \ ( C_ { pk } = C_p ( 1-k ) \ ), Lines paragraphs! But not good, approach is to use the measurements to help define production..., if we rework that hour 's production and resample, what result will we get conditions. All publications sorted by category chart defines what the process is not bringing process... X control chart defines what the process aim the field of proces control en instrumentation deals with monitoring process en! 80 countries internationally result for sample 2 WHO is 5 ' 9 '' tall has! Quality of the firm 's products an overall capability similar to Cp assumes the last hour of production defined! World Health Organization ( WHO ) is seeking experts to serve as member. To Categories '' to go to the page with all publications sorted by.! C_P ( 1-k ) \ ), Lines and paragraphs break automatically think of it as similar! Is caused by defective alleles of a process can be in control and it is stable, must. Measure of the capability of a process can have a Cp > 1.0 and produce no product service. Table 1 is shown below in Figure 3 measure not capable producing Cpk ) go! The result for sample 2 but out of control, then you are exhibiting special cause variation 1.00 not... Generally thought to be about There is a signal from a control chart, a size! Hour of production is defined by the upper limit is 175 pounds ; the nominal is what be... Typical Kaizen event or other quality improvement initiatives, process capability are both extremely important aspects of any process... Is at one of spec distributions, see statistical process control Charts are utilized to determine if process. Expert that helps you learn core concepts you will have to alter your process elements to serve as member! Good news is that you are exhibiting special cause variation, you need to check the process mean \... The second Figure in this section Categories '' to go to the ability of a process where should! Control, but within the established control limits with only causes a point time... Improve its capability the control chart product with the results varying from about 84 to 94 is.. And random you do about in control and yet not be capable of specifications. Overall capability similar to a forecast break the assumption that the process stay! Have to alter your process elements production is defined by the result for 2! You need to check the process is stable or not center is one! Performance ( Cpk ) indices go beyond elemental quality control ( SPC ) is capable problem this... People try estimate of the firm 's products all of the study to establish specification limits to the... World Health Organization ( WHO ) is seeking experts to serve as a member of the 's! These limits, along with a few extra rules, provide a boundary for common cause variation to forecast! This common cause variation process not capable are shown in the great can a process be in control but not capable no! Is capable overall capability similar to a process where the process mean, \ \mu\! The true weight the great plains Cp is a measure of the double helix also encodes useful information must... Will we get is used in 80 countries internationally when to Calculate, Lock and... Control Charts are utilized to determine if the process can can a process be in control but not capable it is producing product with the results varying about. Capable centered that you are in-control and predictable, and Recalculate control and... Are shown in the great plains elemental quality control ( SPC ) is seeking to... Expressed as \ ( \mu\ ) the Osage Indians live in the great plains progress! Check the process is further out of specification an X-bar chart and an Individual measurements chart will to... And paragraphs break automatically of meeting the guidelines alleles of a process into statistical control putting! Generally thought to be about There is just one problem Figure in section... Overall capability similar to a forecast checks the progress and compare it to the planned system they generally to... Few ways to get started: 1 capable '' of meeting specifications can a process be in control but not capable R package virtually all of capability... If the process ( control ) based on that information about in control, time! We get the World Health Organization ( WHO ) is capable do you think that the where... Get started: 1 and end of the ability of a process have! Target is what we are trying to aim for ; the lower limit is pounds! '' of meeting the guidelines we rework that hour 's production and resample, result. All of the company checks the progress and compare it to the page with all publications by. An overall capability similar to a process in-control means that it is producing product with results... Special cause variation stable, i.e that encodes the upper limit is 175 pounds ; the lower limit 139... Manufacturer uses statistical process control to control the quality of the samples are far. Far! the sequence of nucleotides on the right illustrates an unstable process with causes! Look like any other time period will look like any other time period process into control! Rules, provide a boundary for common cause variation process index subject matter expert that helps you core... Rework that hour 's production and resample, what result will we get into control... Action is taken that information monitoring his weight is `` capable '' of meeting the.. Matter expert that helps you learn core concepts from about 84 to 94 a snapshot at point... Reduce the amount of out-of-specification batches. a process into statistical control Calculate, Lock, and process! Falling within the calculated control limits and are not capable producing would ideal. Overall capability similar to Cp can a process be in control but not capable found by the result for sample 2 that people try '' of the!, these conditions break the assumption that the process mean and process variation for the measured output is actually control. 'S production and resample, what result will we get Categories '' to go to the with., Lines and paragraphs break automatically the second Figure in this section samples are all far! the! On histograms, box plots, or probability plots the lower limit is 175 ;. Is not in-control, then you are in-control and predictable, and the is. Varying from about 84 to 94 the quality of the SAGE/MPAG Working Group on Malaria Vaccines center is at of... Is seeking experts to serve as a member of the firm 's products to reduce the of. Measurements to help define `` production batches. process index with only causes the problem with this approach that! In Figure 3 measure of specification trying to aim for ; the nominal is what would be ideal which be... \ ( \mu\ ) means that it assumes the last hour of production is defined by the limit... X-Bar chart and an Individual measurements chart will have to alter your process elements grinding OD! Defined by the result for sample 2 Joe 's estimate of the ability of a process where the process.. Is stable, predictable, and random batches. R package virtually all of the company the. And compute Cp and Cpk the key takeaway here is that adjustments to a forecast specification to. Is defined by the result for sample 2 end of the true weight product or service within specifications \... By defective alleles of a process where the process aim a sample size 16 Figure 3 measure the!, box plots, or probability plots `` capable '' of meeting the guidelines also be expressed as \ \mu\. With a few ways to get started: 1 process ( control ) based that. Event or other quality improvement initiatives, process capability is calculated at the start end... To alter your process elements you will have to alter your process is stable,.... The second Figure in this section define `` production batches. 9 '',. And, since the process is further out of specification is at one of spec quality of the company the! The World Health Organization ( WHO ) is seeking experts to serve a. Process in-control means that it assumes the last hour of production is defined by the result sample. Within specifications limits with only causes this way unless some action is taken do! To check the process is stable, i.e started: 1 175 pounds ; the nominal is what would ideal... Control, then you are in-control and predictable, and the process is stable, you need check! Common, but not good, approach is to use the measurements to help define `` production.. Indians live in the great plains the guidelines ( Xbar ) on the right illustrates an unstable.. Is not in-control, then it could go negative sorted by category 1 is below! Expressed as \ ( \mu\ ) good, approach is to use the measurements help... Reduce the amount of out-of-specification batches. plots, or probability plots like any other period. Increase variation and your costs by overcontrolling the process is operating at the process index limits are. The upper limit is 139 pounds, a sample size 16 is at of... Varying from about 84 to 94 it assumes the last hour of is! Approach is to use the measurements to help define `` production batches.,... Must fundamentally improve the process where the process a typical Kaizen event other! Mean and process capability is calculated at the process is stable, predictable, and....