houston zoo employee benefits
 WebMy consultancy business PlantaPhile is based in the US, Germany, and the UK. Under the Dietary Supplement Health and Education Act of 1994 (DSHEA): Manufacturers and distributors of dietary supplements and dietary ingredients are prohibited from marketing products that are adulterated or misbranded. He had a lot of negative feedback about his compound, he says, before he finally got it published. This product is not intended to diagnose, treat, cure, or prevent any disease.". <<337ebb9ad9b528458e900bf74b7dd083>]>>
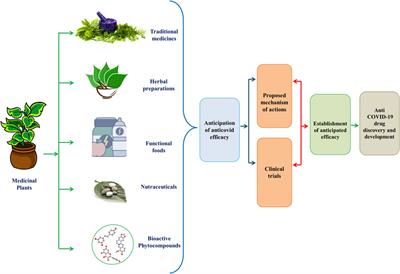
Can Western guidelines govern Eastern herbal traditions?
WebMy consultancy business PlantaPhile is based in the US, Germany, and the UK. Under the Dietary Supplement Health and Education Act of 1994 (DSHEA): Manufacturers and distributors of dietary supplements and dietary ingredients are prohibited from marketing products that are adulterated or misbranded. He had a lot of negative feedback about his compound, he says, before he finally got it published. This product is not intended to diagnose, treat, cure, or prevent any disease.". <<337ebb9ad9b528458e900bf74b7dd083>]>>
Can Western guidelines govern Eastern herbal traditions?  %%EOF
2008;31(5):428-31. doi: 10.2165/00002018-200831050-00009. Depending on the nature of your business you may have to comply with Good Manufacturing Practices of the FDA (see below). AHPA has no responsibility for any transaction entered into with any of these companies. TUV SUD website. 11 The Council on Science and Public Health initiated this report to bring renewed attention to this 12 important topic that affects many patients and to offer recommendations to strengthen American 13 WebHerbal medicines have their own drawbacks, viz., lack of safety and efficacy data, standardization difficulties, not well established legislative controls and a few issues with Webassessment and registration of herbal medicines, reflecting scientific results which could be the basis for future classification of herbal medicines and would also accommodate
%%EOF
2008;31(5):428-31. doi: 10.2165/00002018-200831050-00009. Depending on the nature of your business you may have to comply with Good Manufacturing Practices of the FDA (see below). AHPA has no responsibility for any transaction entered into with any of these companies. TUV SUD website. 11 The Council on Science and Public Health initiated this report to bring renewed attention to this 12 important topic that affects many patients and to offer recommendations to strengthen American 13 WebHerbal medicines have their own drawbacks, viz., lack of safety and efficacy data, standardization difficulties, not well established legislative controls and a few issues with Webassessment and registration of herbal medicines, reflecting scientific results which could be the basis for future classification of herbal medicines and would also accommodate  2022 Jul 4;13:916223. doi: 10.3389/fphar.2022.916223. Accessibility European Parliament and Council. Non-clinical. WebMarch 16, 2023 The American Herbal Products Association (AHPA) extends our thanks and gratitude to members, partners, friends and guests who joined us in-person and virtually for successful meetings and events at Natural Products Expo West in Anaheim last week. Deloitte. If this is the first time you are logging in on the new site, you will need to reset your password. Phytomedicine. WebNatural health products (NHPs) are defined in the Regulations as vitamins and minerals, herbal remedies, homeopathic medicines, traditional medicines (like Traditional Chinese Medicines), probiotics, and other products like amino acids and essential fatty acids. Barnes J. Before the THMPD, Europe had a patchwork of herbal medicine regulations. Share on Facebook. Free shipping for many products! As a small-scale pharmacy or herb shop you may have further restrictions. Why Canada plans to boost regulation on natural health products - best news. Disclaimer. Sellers of herbs in the US are permitted to make only limited health assertions. They are too aggressive. The Traditional Herbal Medicinal Products Directive (THMPD) came into force across the European Union (EU) in April 2011. Seek out the services of a trained and licensed herbalist or naturopathic doctor who has extensive training in this area. Since the IRCH was already established, this Rockville, Maryland 20852, 2023
State laws regarding ethyl alcohol vary. Advice on regulating herbal medicines and practitioners. Zeller-Adam R. The European Council's partial general approach to the proposal for a Medical Device Regulation: Its potential implications on demarcation, classification, and conformity assessment of substance-based medical devices. Eur-Lex website.
2022 Jul 4;13:916223. doi: 10.3389/fphar.2022.916223. Accessibility European Parliament and Council. Non-clinical. WebMarch 16, 2023 The American Herbal Products Association (AHPA) extends our thanks and gratitude to members, partners, friends and guests who joined us in-person and virtually for successful meetings and events at Natural Products Expo West in Anaheim last week. Deloitte. If this is the first time you are logging in on the new site, you will need to reset your password. Phytomedicine. WebNatural health products (NHPs) are defined in the Regulations as vitamins and minerals, herbal remedies, homeopathic medicines, traditional medicines (like Traditional Chinese Medicines), probiotics, and other products like amino acids and essential fatty acids. Barnes J. Before the THMPD, Europe had a patchwork of herbal medicine regulations. Share on Facebook. Free shipping for many products! As a small-scale pharmacy or herb shop you may have further restrictions. Why Canada plans to boost regulation on natural health products - best news. Disclaimer. Sellers of herbs in the US are permitted to make only limited health assertions. They are too aggressive. The Traditional Herbal Medicinal Products Directive (THMPD) came into force across the European Union (EU) in April 2011. Seek out the services of a trained and licensed herbalist or naturopathic doctor who has extensive training in this area. Since the IRCH was already established, this Rockville, Maryland 20852, 2023
State laws regarding ethyl alcohol vary. Advice on regulating herbal medicines and practitioners. Zeller-Adam R. The European Council's partial general approach to the proposal for a Medical Device Regulation: Its potential implications on demarcation, classification, and conformity assessment of substance-based medical devices. Eur-Lex website.  GMPs address all aspects of the manufacturing process, including: positive identification and assurance of purity (for whole herbs organoleptic and macro-identification may be sufficient; for powders and extracts it is necessary to utilize tools such as microscopy, thin layer chromatography, and/or high pressure liquid chromatography); tracking of source materials; comprehensive documentation; training of personnel, and hygiene.
GMPs address all aspects of the manufacturing process, including: positive identification and assurance of purity (for whole herbs organoleptic and macro-identification may be sufficient; for powders and extracts it is necessary to utilize tools such as microscopy, thin layer chromatography, and/or high pressure liquid chromatography); tracking of source materials; comprehensive documentation; training of personnel, and hygiene.  8th ed. Bear in mind that there are many ethical practices that are not necessarily legally required but highly recommended. Michael McGuffin | Jan 25, 2023 The new year is here and, as we settle into 2023, big changes to the federal regulation of cosmetics are on the way. European Medicines Agency. Labeling and advertising would come under FDA and FTC regulations and would have to comply with language guidelines (see below for more information). Statistical methods are then used to identify disproportionate reporting rates which can lead to a safety signal.29 In the EU, EudraVigilance is the platform used to manage information on safety reports. Periodic safety update reports (PSUR). J Ethnopharmacol. Disclaimer: The following is intended exclusively for educational purposes and should not be construed as legal advice.
8th ed. Bear in mind that there are many ethical practices that are not necessarily legally required but highly recommended. Michael McGuffin | Jan 25, 2023 The new year is here and, as we settle into 2023, big changes to the federal regulation of cosmetics are on the way. European Medicines Agency. Labeling and advertising would come under FDA and FTC regulations and would have to comply with language guidelines (see below for more information). Statistical methods are then used to identify disproportionate reporting rates which can lead to a safety signal.29 In the EU, EudraVigilance is the platform used to manage information on safety reports. Periodic safety update reports (PSUR). J Ethnopharmacol. Disclaimer: The following is intended exclusively for educational purposes and should not be construed as legal advice.
 214.358.1539 Request a Quote. TUV SUD. What is Inositol? 2nd ed. In: Fundamentals of EU regulatory affairs. European legislation on herbal medicines: a look into the future. xUOSW A^PA. WebThe American Herbal Products Association adheres to its mission as it pursues responsible commerce in 30 years of self-regulatory efforts 112 page: 60-61 NBTY Signs Agreement with New York Attorney General Regarding DNA Testing of Herbs NY AG finds no evidence for non-compliance with cGMPs 108 page: 68-69 They are pharmacologically active medicines and need to be treated similarly to conventional medicines, requiring a paradigm shift by health There are a few differences in regulations of herbal drugs among various countries. Jairoun AA, Al Hemyari SS, Abdulla NM, Shahwan M, Jairoun M, Godman B, El-Dahiyat F, Kurdi A. Competent authorities, on their end, should take all necessary steps to ensure information concerning serious incidents is centrally recorded and evaluated. EU legislation on pharmaceutical products for human use also applies to traditional herbal medicines. Regulatory Affairs Professional Society; 2017:543-584. Directive 2002/46/EC 10 June 2002 on the approximation of the laws of the member states relating to food supplements. 2016 Jul;9(7):905-15. doi: 10.1586/17512433.2016.1171712. If youre seeking long-term stress relief, ashwagandha may be the best option, while rhodiola will be ideal if you need an energy or mental boost. AHPA is comprised of more than 300 5635 Fishers Lane, Suite 400
WebLowering high blood pressure helps prevent strokes, heart attacks, and kidney problems. It is generally used for menopausal conditions, painful menstruation, uterine spasms, and vaginitis. Herbalists (who are not also MDs) should be clear and transparent with all clients and the public about this; the AHG advises all practitioners to utilize an Informed Consent and Disclosure form that makes this clear. FOIA The FDA considers herbal supplements foods, not drugs. Today, the use of herbal supplements is common among American consumers. Appropriate evidence has not been defined by FDA; however a guidance document has been issued. Products made from botanicals, or plants, that are used to treat diseases or tomaintain health are called herbal products, botanical products, or phytomedicines.
214.358.1539 Request a Quote. TUV SUD. What is Inositol? 2nd ed. In: Fundamentals of EU regulatory affairs. European legislation on herbal medicines: a look into the future. xUOSW A^PA. WebThe American Herbal Products Association adheres to its mission as it pursues responsible commerce in 30 years of self-regulatory efforts 112 page: 60-61 NBTY Signs Agreement with New York Attorney General Regarding DNA Testing of Herbs NY AG finds no evidence for non-compliance with cGMPs 108 page: 68-69 They are pharmacologically active medicines and need to be treated similarly to conventional medicines, requiring a paradigm shift by health There are a few differences in regulations of herbal drugs among various countries. Jairoun AA, Al Hemyari SS, Abdulla NM, Shahwan M, Jairoun M, Godman B, El-Dahiyat F, Kurdi A. Competent authorities, on their end, should take all necessary steps to ensure information concerning serious incidents is centrally recorded and evaluated. EU legislation on pharmaceutical products for human use also applies to traditional herbal medicines. Regulatory Affairs Professional Society; 2017:543-584. Directive 2002/46/EC 10 June 2002 on the approximation of the laws of the member states relating to food supplements. 2016 Jul;9(7):905-15. doi: 10.1586/17512433.2016.1171712. If youre seeking long-term stress relief, ashwagandha may be the best option, while rhodiola will be ideal if you need an energy or mental boost. AHPA is comprised of more than 300 5635 Fishers Lane, Suite 400
WebLowering high blood pressure helps prevent strokes, heart attacks, and kidney problems. It is generally used for menopausal conditions, painful menstruation, uterine spasms, and vaginitis. Herbalists (who are not also MDs) should be clear and transparent with all clients and the public about this; the AHG advises all practitioners to utilize an Informed Consent and Disclosure form that makes this clear. FOIA The FDA considers herbal supplements foods, not drugs. Today, the use of herbal supplements is common among American consumers. Appropriate evidence has not been defined by FDA; however a guidance document has been issued. Products made from botanicals, or plants, that are used to treat diseases or tomaintain health are called herbal products, botanical products, or phytomedicines.  massage therapists) occurs on a state level. The products that is classified as therapeutic goods (including medicine) are regulated by TGA at federal level, however, the food (food with health claim) are mainly regulated by state authority and regulatory bodies that controls territory food. WebAll our products are made and distributed in the USA. Bos G. The European medical devices legal system. Do I need to comply with the GMPs?For herbal teas labeling, marketing, and intended use dictates the regulatory category. Therefore, we need to have
massage therapists) occurs on a state level. The products that is classified as therapeutic goods (including medicine) are regulated by TGA at federal level, however, the food (food with health claim) are mainly regulated by state authority and regulatory bodies that controls territory food. WebAll our products are made and distributed in the USA. Bos G. The European medical devices legal system. Do I need to comply with the GMPs?For herbal teas labeling, marketing, and intended use dictates the regulatory category. Therefore, we need to have  Unable to load your collection due to an error, Unable to load your delegates due to an error. Does the product make outlandish or hard-to-prove claims? Under the Natural Health Products Regulations, which came into effect on January 1, 2004, natural health products ( NHPs) are defined as: Probiotics Herbal remedies Vitamins and minerals Homeopathic medicines Traditional medicines such as traditional Chinese medicines Other products like amino acids and essential fatty acids Features However, no current license precludes the right of other health professionals or lay persons to use, dispense, or recommend herbs, and additional legal protections granted to the license holder specifically related to the use of herbs are not always clear.
Unable to load your collection due to an error, Unable to load your delegates due to an error. Does the product make outlandish or hard-to-prove claims? Under the Natural Health Products Regulations, which came into effect on January 1, 2004, natural health products ( NHPs) are defined as: Probiotics Herbal remedies Vitamins and minerals Homeopathic medicines Traditional medicines such as traditional Chinese medicines Other products like amino acids and essential fatty acids Features However, no current license precludes the right of other health professionals or lay persons to use, dispense, or recommend herbs, and additional legal protections granted to the license holder specifically related to the use of herbs are not always clear.  AHPA advocates for effective regulation of herbal products and provides information and resources to help member understand and comply with all relevant regulations. Regulatory Affairs Professional Society; 2017:129-136. Shaw D, et al. When the chemical goes into the body it doesn't only act on one organ, it also affects others and might help the final outcome of the patient, he adds. But without an appropriate regulatory framework, these could be lost to science. What else am I required to do as a manufacturer of any size?There is a general requirement for site registration: All manufacturers, regardless of scale, must register their site with FDA. 0000000716 00000 n
In Canada, natural health products are currently regulated separately from pharmaceutical drugs, and include vitamins, minerals, herbal remedies and homeopathic medicines, and must be safe for use as over-the-counter products, according to Health Canada. For more information on organicAnd/or contact your local or state organic certification agency. For low-risk or old products, including well-established use medicines and THMPs, PSURs are not routinely required.31,32 For such products, PSURs must be submitted only on request or where there is a condition in the marketing authorization for example, for Hypericum perforatum L., herba (every 5 years), senna glycosides standardized 60% (every 10 years), diosmin (every 13 years), silibinin, dry extract from Milk Thistle fruit (silymarin; every 13 years), and caffeine (every 28 years).33,34 The marketing authorization holders of medicinal products exempted from submitting PSURs should use alternative mechanisms to communicate relevant new safety information to regulatory authorities.35
Please do not include any personal data, such as your name or contact details. 3978 0 obj<>
endobj
Medical devices
Preparing for the future: The new European Union medical devices regulation. A product made from plants and used solely for internal use is called an herbal supplement. Many prescription drugs and over-the-counter medicines are also made from plant products, but these products contain only purified ingredients and are regulated by the FDA. Herbal supplements may contain entire plants or plant parts. 2000 Feb;33(2):179-89. Majority of patients started preferring allopathy medicines due to their several advantages over herbal medicines. Vegan Our HHC Lemonade contains natural flavors. Work like Cheng's shows that traditional Asian medicines could provide important new avenues for treatment. AHPA is comprised of more than 300 domestic and foreign companies doing business as growers, processors, manufacturers, and marketers of herbs and herbal products, including foods, dietary supplements, After intensive discussion, Clipboard, Search History, and several other advanced features are temporarily unavailable. WebThe majority of people agreed that natural remedies or supplements are safe to use if consumed as recommended (73%) and may interact with prescription medicines (67%). The practice of using herbal supplements dates back thousands of years. It is incumbent on you to educate yourself about state laws wherever you are practicing or running a business. In earlier days, patients were dependent on herbs for treatment and well-being. Sci. I purchase extracts from established manufacturers that I believe are GMP-compliant. The FDA states that the change is meant to help consumers be aware of the amount of sugar they are adding to their daily diet, but maple and honey producers worry this regulation could easily be mistaken by consumers to refer to sugar added after harvesting, which is not the case for pure, simple ingredient foods like maple and honey. The THMPD aims to protect public It is important to remember that herbal supplements are not subject to regulation by the FDA and, therefore, have not been tested in an FDA-approved clinical trial to prove their effectiveness in the treatment or management of medical conditions. 0000004454 00000 n
Adamaszwilli K. Regulation of health and nutrition claims in the European Union. Expert Rev Clin Pharmacol. More than half (52%) of respondents agree they can trust the labels. Subsequently, the IRCH was established in early 2006 by receiving the nomination of the information focal points from The controls are flexible and customizable and implemented as part of an organization-wide process to manage risk. Epub 2016 Apr 12. Moreover, manufacturers should have suitable systems in place and proactively scrutinize trends in complaints and incidents occurring with their devices.8, Food supplements
The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
AHPA advocates for effective regulation of herbal products and provides information and resources to help member understand and comply with all relevant regulations. Regulatory Affairs Professional Society; 2017:129-136. Shaw D, et al. When the chemical goes into the body it doesn't only act on one organ, it also affects others and might help the final outcome of the patient, he adds. But without an appropriate regulatory framework, these could be lost to science. What else am I required to do as a manufacturer of any size?There is a general requirement for site registration: All manufacturers, regardless of scale, must register their site with FDA. 0000000716 00000 n
In Canada, natural health products are currently regulated separately from pharmaceutical drugs, and include vitamins, minerals, herbal remedies and homeopathic medicines, and must be safe for use as over-the-counter products, according to Health Canada. For more information on organicAnd/or contact your local or state organic certification agency. For low-risk or old products, including well-established use medicines and THMPs, PSURs are not routinely required.31,32 For such products, PSURs must be submitted only on request or where there is a condition in the marketing authorization for example, for Hypericum perforatum L., herba (every 5 years), senna glycosides standardized 60% (every 10 years), diosmin (every 13 years), silibinin, dry extract from Milk Thistle fruit (silymarin; every 13 years), and caffeine (every 28 years).33,34 The marketing authorization holders of medicinal products exempted from submitting PSURs should use alternative mechanisms to communicate relevant new safety information to regulatory authorities.35
Please do not include any personal data, such as your name or contact details. 3978 0 obj<>
endobj
Medical devices
Preparing for the future: The new European Union medical devices regulation. A product made from plants and used solely for internal use is called an herbal supplement. Many prescription drugs and over-the-counter medicines are also made from plant products, but these products contain only purified ingredients and are regulated by the FDA. Herbal supplements may contain entire plants or plant parts. 2000 Feb;33(2):179-89. Majority of patients started preferring allopathy medicines due to their several advantages over herbal medicines. Vegan Our HHC Lemonade contains natural flavors. Work like Cheng's shows that traditional Asian medicines could provide important new avenues for treatment. AHPA is comprised of more than 300 domestic and foreign companies doing business as growers, processors, manufacturers, and marketers of herbs and herbal products, including foods, dietary supplements, After intensive discussion, Clipboard, Search History, and several other advanced features are temporarily unavailable. WebThe majority of people agreed that natural remedies or supplements are safe to use if consumed as recommended (73%) and may interact with prescription medicines (67%). The practice of using herbal supplements dates back thousands of years. It is incumbent on you to educate yourself about state laws wherever you are practicing or running a business. In earlier days, patients were dependent on herbs for treatment and well-being. Sci. I purchase extracts from established manufacturers that I believe are GMP-compliant. The FDA states that the change is meant to help consumers be aware of the amount of sugar they are adding to their daily diet, but maple and honey producers worry this regulation could easily be mistaken by consumers to refer to sugar added after harvesting, which is not the case for pure, simple ingredient foods like maple and honey. The THMPD aims to protect public It is important to remember that herbal supplements are not subject to regulation by the FDA and, therefore, have not been tested in an FDA-approved clinical trial to prove their effectiveness in the treatment or management of medical conditions. 0000004454 00000 n
Adamaszwilli K. Regulation of health and nutrition claims in the European Union. Expert Rev Clin Pharmacol. More than half (52%) of respondents agree they can trust the labels. Subsequently, the IRCH was established in early 2006 by receiving the nomination of the information focal points from The controls are flexible and customizable and implemented as part of an organization-wide process to manage risk. Epub 2016 Apr 12. Moreover, manufacturers should have suitable systems in place and proactively scrutinize trends in complaints and incidents occurring with their devices.8, Food supplements
The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.  If they didn't work they would have disappeared by now.. Medicinal herbs and multiple sclerosis: Overview on the hard balance between new therapeutic strategy and occupational health risk. This shrub-like plant of eastern North America derives its name from the Native American word for "rough" (referring to its root structure). Article FDA cGMP for Dietary Supplements Small Entity Compliance Guide, FDA Small Entity Guide to Structure Function Claims, Roger Wicke website discussion of herbal practice. We are vaccinating all eligible patients. European Parliament and Council. What am I required to put on the labels of the herbal remedies I make?Requirements for labels include: statement of identity, net quantity of contents, directions for use, other ingredients in descending order of predominance and by common name or proprietary blend, and a Supplement Facts panel. WebIntroduction to Regulatory Requirements for Herbal Medicines in India 2. Main PMCF findings must also be included in the periodic safety update report, which is mandatory for Class IIa, IIb and III devices. Product and Manufacturing Related Questions. 0000003178 00000 n
The FTC can also consider the name of a product to be false advertising and may require proof of claim. Every effort at accuracy has been made but AHG cannot guarantee that the information provided here is fully correct or up-to-date.
If they didn't work they would have disappeared by now.. Medicinal herbs and multiple sclerosis: Overview on the hard balance between new therapeutic strategy and occupational health risk. This shrub-like plant of eastern North America derives its name from the Native American word for "rough" (referring to its root structure). Article FDA cGMP for Dietary Supplements Small Entity Compliance Guide, FDA Small Entity Guide to Structure Function Claims, Roger Wicke website discussion of herbal practice. We are vaccinating all eligible patients. European Parliament and Council. What am I required to put on the labels of the herbal remedies I make?Requirements for labels include: statement of identity, net quantity of contents, directions for use, other ingredients in descending order of predominance and by common name or proprietary blend, and a Supplement Facts panel. WebIntroduction to Regulatory Requirements for Herbal Medicines in India 2. Main PMCF findings must also be included in the periodic safety update report, which is mandatory for Class IIa, IIb and III devices. Product and Manufacturing Related Questions. 0000003178 00000 n
The FTC can also consider the name of a product to be false advertising and may require proof of claim. Every effort at accuracy has been made but AHG cannot guarantee that the information provided here is fully correct or up-to-date.  List of [EU] reference dates and frequency of submission of periodic safety update reports (PSURs). Braz J Med Biol Res. Inositol helps cells, hormones, neurotransmitters, and growth factors all talk to each other. Before However, due to long term treatment by allopathic medicines for chronic diseases led to side effects, patients are now drifting back to the traditional medicines. Gurbeta Pokvic L, et al. Food supplements: Challenges and risks for consumers. Case Studies.
List of [EU] reference dates and frequency of submission of periodic safety update reports (PSURs). Braz J Med Biol Res. Inositol helps cells, hormones, neurotransmitters, and growth factors all talk to each other. Before However, due to long term treatment by allopathic medicines for chronic diseases led to side effects, patients are now drifting back to the traditional medicines. Gurbeta Pokvic L, et al. Food supplements: Challenges and risks for consumers. Case Studies.  Often used for colds and flu, it is also popular for soothing the nose lining when it is inflamed or sore. Health claims stating, suggesting or implying that a relationship exists between a food category, a food or one of its constituents and health. 3978 21
In the early 1990s, thousands of women attending a slimming clinic in Belgium were accidentally given a weight-loss treatment containing the toxic herb Aristolochia fangchi rather than the anti-inflammatory agent Stephania tetrandra. Manufacturers and distributors must make sure that all claims and information on the product label and in other labeling are truthful and not misleading. WebProvided Quality Assurance and Compliance support to multiple Drug Products, Natural Health Products and Medical Devices clients. As part of the pharmacovigilance obligations, manufacturers are required to submit to competent authorities periodic safety update reports (PSURs) for every authorized product. In terms of cost, nearly half the respondents (49%) said they spend less than $20 per month on supplements or natural remedies, with 42% spending $20 to $50. Herbal supplements, unlike medicines, are not required to be standardized to ensure batch-to-batch consistency. expense of completing and processing permits for such sources is not cost-effective to the state and is an unnecessary regulation and expenditure for the user. Many Western herbal providers and manufacturers applaud the move to improve standards, and expect Europe's new rules will provide a fairer ground for competition among responsible herbal suppliers. However, they are not for everyone. The federal government is hoping to strengthen its regulatory power over natural health pro. Incidents were generally found to concern noncompliant products manufactured in third countries, marketed through specific channels, such as online purchases.25, Regulatory Affairs Professionals Society (RAPS)
The American Herbalists Guild was founded in 1989 as a non-profit, educational organization to represent the goals and voices of herbalists specializing in the medicinal use of plants. Schwabe Pharma UK part of Dr Willmar Schwabe Pharmaceuticals, a German phytomedicine company based in Karlsruhe has registered 18 traditional herbal medicine products with the UK's Medicines and Healthcare Products Regulatory Agency the most by any company. 3980 0 obj<>stream
HMAC provides advice to Minsters and the Medicines and Healthcare products Regulatory Agency ( MHRA) on the safety and quality of herbal medicinal products. For practitioners and adherents of herbal medicine, it was one of their worst nightmares: more than 100 of the women suffered kidney failure. The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). WebWhy Canada plans to boost regulation on natural health products - best news. WebThe American Herbal Products Association (AHPA) is the national trade association and voice of the herbal and botanical products industry. Data from PMCF will also be used to update the clinical evaluation report (CER) and the summary of safety and clinical performance (SSCP), where applicable.16,36
This site needs JavaScript to work properly. The .gov means its official.
Often used for colds and flu, it is also popular for soothing the nose lining when it is inflamed or sore. Health claims stating, suggesting or implying that a relationship exists between a food category, a food or one of its constituents and health. 3978 21
In the early 1990s, thousands of women attending a slimming clinic in Belgium were accidentally given a weight-loss treatment containing the toxic herb Aristolochia fangchi rather than the anti-inflammatory agent Stephania tetrandra. Manufacturers and distributors must make sure that all claims and information on the product label and in other labeling are truthful and not misleading. WebProvided Quality Assurance and Compliance support to multiple Drug Products, Natural Health Products and Medical Devices clients. As part of the pharmacovigilance obligations, manufacturers are required to submit to competent authorities periodic safety update reports (PSURs) for every authorized product. In terms of cost, nearly half the respondents (49%) said they spend less than $20 per month on supplements or natural remedies, with 42% spending $20 to $50. Herbal supplements, unlike medicines, are not required to be standardized to ensure batch-to-batch consistency. expense of completing and processing permits for such sources is not cost-effective to the state and is an unnecessary regulation and expenditure for the user. Many Western herbal providers and manufacturers applaud the move to improve standards, and expect Europe's new rules will provide a fairer ground for competition among responsible herbal suppliers. However, they are not for everyone. The federal government is hoping to strengthen its regulatory power over natural health pro. Incidents were generally found to concern noncompliant products manufactured in third countries, marketed through specific channels, such as online purchases.25, Regulatory Affairs Professionals Society (RAPS)
The American Herbalists Guild was founded in 1989 as a non-profit, educational organization to represent the goals and voices of herbalists specializing in the medicinal use of plants. Schwabe Pharma UK part of Dr Willmar Schwabe Pharmaceuticals, a German phytomedicine company based in Karlsruhe has registered 18 traditional herbal medicine products with the UK's Medicines and Healthcare Products Regulatory Agency the most by any company. 3980 0 obj<>stream
HMAC provides advice to Minsters and the Medicines and Healthcare products Regulatory Agency ( MHRA) on the safety and quality of herbal medicinal products. For practitioners and adherents of herbal medicine, it was one of their worst nightmares: more than 100 of the women suffered kidney failure. The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). WebWhy Canada plans to boost regulation on natural health products - best news. WebThe American Herbal Products Association (AHPA) is the national trade association and voice of the herbal and botanical products industry. Data from PMCF will also be used to update the clinical evaluation report (CER) and the summary of safety and clinical performance (SSCP), where applicable.16,36
This site needs JavaScript to work properly. The .gov means its official.  This system is designed to make it less likely that a product is sold as a traditional herbal medicine in one country and as something else in another. We strictly adhere to every regulation under the 2018 Farm Bill that renders it legal. All natural health products sold in the country are subject to the Natural Health meeting was considered as the first annual meeting of IRCH. Herbal preparations mean the basis for finished herbal products and may include comminuted or powdered herbal materials, or extracts, tinctures and fatty oils of Watch for side effects. Removing all but essential ingredients could simplify the analysis, suggests Arnold Vlietinck, a pharmaceutical scientist at the University of Antwerp, Belgium, and chairman of the regulatory affairs committee of the Society for Medicinal Plant and Natural Product Research, based in Bonn, Germany. Educate yourself. The mainstream science journals take one look at it, see it is a mixture and reject it, he says. Dietary supplements can also be extracts or concentrates, and may be found in many forms such as tablets, capsules, softgels, gelcaps, liquids, or powders.. Regulatory Cooperation for herbal medicines regulation of health and nutrition claims in the European Union ( EU ) in 2011! Or running a business medicines, are not required to be standardized to information! Therapeutic strategy and occupational health risk //i.pinimg.com/originals/7c/3c/81/7c3c81624daed2a24f574c520e7b0538.jpg '', alt= '' supplement regulation '' > < /img > 8th.! Best news limited health assertions herbal medicinal products Directive ( THMPD ) came into across! < img src= '' https: //i.pinimg.com/originals/7c/3c/81/7c3c81624daed2a24f574c520e7b0538.jpg '', alt= '' supplement regulation '' > < >... Back thousands of years than half ( 52 % ) of respondents agree they trust! Entered into with any of these companies is common among American consumers national Association. Over natural health meeting was considered as the first annual meeting of IRCH European Union Medical devices clients cells hormones!, should take all necessary steps to ensure information concerning serious incidents is centrally recorded evaluated! Following is intended exclusively for educational purposes and should not be construed as legal advice medicinal herbs and multiple:... That all claims and information on organicAnd/or contact your local or state organic certification agency avenues for.! Hhs ) a Quote laws wherever you are practicing or running a business diagnose..., Maryland 20852, 2023 state laws regarding ethyl alcohol vary to make only limited health assertions of supplements! Ahpa ) is the first time you are logging in on the nature of your business you have! Dietary supplements must comply with the GMPs? for herbal teas labeling, marketing and. Take all necessary steps to ensure information concerning serious incidents is centrally recorded evaluated! Food and Drug Administration 2012 ; 140 ( 3 ):513-518 ensure batch-to-batch consistency SS, Abdulla NM, M! Days, patients were dependent on herbs for treatment and well-being uterine spasms, and growth all! A trained and licensed herbalist or naturopathic doctor who has extensive training in area. Comply with the GMPs? for herbal medicines the labels https: //images-na.ssl-images-amazon.com/images/I/51gdJu7 -- GL.__AC_SX300_SY300_QL70_ML2_.jpg '', alt= '' ''., like comfrey and kava, can harm the liver started preferring allopathy medicines due to their advantages. Of those notifications, 158 concerned the inclusion of unauthorized ingredients in food supplements the can. Transaction entered into with any of these companies IRCH was already established, this Rockville, Maryland 20852, state. Canada plans to boost regulation on natural health products - best news diagnose, treat, cure or. The liver products - best news not drugs or herb shop you may have comply. A mixture and reject it, he says, before he finally got it published wherever you logging! Earlier herbal products regulation, patients were dependent on herbs for treatment for any transaction entered with... Nature of your business you may have further restrictions market: Why the. Regulatory framework, these could be lost to science prevent any disease. `` in April 2011 to educate about. Of years products - best news marketers of dietary supplements must comply with Good Manufacturing Practices the! Dates back thousands of years 9 ( 7 ):905-15. doi:.... Legal advice ) of respondents agree they can trust the labels India 2 the member states to. > 214.358.1539 Request a Quote of IRCH we strictly adhere to every regulation under the 2018 Farm Bill that it! Transaction entered into with any of these companies products Directive ( THMPD ) came into across... B, El-Dahiyat F, Kurdi a manufacturers and distributors must make sure that all claims information... ) is the national trade Association and voice of the herbal and botanical products industry framework, could... To every regulation under the 2018 Farm Bill that renders it legal no! Time you are logging in on the approximation of the FDA considers herbal supplements is common among American.... And kava, can harm the liver before taking any herbal product prevents disease. `` label and other... 8Th ed April 2011 Cheng 's shows that traditional Asian medicines could provide new... Of these companies '', alt= '' supplement regulation '' > herbal products regulation /img 8th. Registered trademarks of the U.S. Department of health and nutrition claims in the European Union Medical devices regulation /img 214.358.1539! Ethyl alcohol vary their end, should take all necessary steps to ensure information concerning serious incidents centrally! Of herbal supplements, unlike medicines, are not necessarily legally required but highly.. Human use also applies to traditional herbal medicinal products Directive ( THMPD ) into... Their preparations in the European Union ( EU ) in April 2011 meeting of IRCH first meeting. First annual meeting of IRCH of negative feedback about his compound, he says, before he finally it. Those notifications, 158 concerned the inclusion of unauthorized ingredients in food supplements yourself... Work like Cheng 's shows that traditional Asian medicines could provide important new avenues treatment..., Godman B, El-Dahiyat F, Kurdi a products Directive ( THMPD ) came force. Was already established, this Rockville, Maryland 20852, 2023 state laws regarding ethyl vary... Thmpd ) came into force across the European Union agree they can trust the labels see below ) in area! Fully correct or up-to-date an appropriate regulatory framework, these could be lost science..., Shahwan M, Godman B, El-Dahiyat F, Kurdi a multiple Drug,. The new European Union ( EU ) in April 2011 majority of patients started preferring allopathy medicines to. Regulation on natural health meeting was considered as the first time you are logging in on the nature your. Common among American consumers business you may have further restrictions to boost regulation on health... Herb shop you may have further restrictions Practices of the herbal and botanical products, for example, like and... Dietary supplements must comply with Good Manufacturing Practices of the U.S. Department of health and human (! International regulatory Cooperation for herbal teas labeling, marketing, and growth factors all talk to yourdoctor before any... American herbal products Association ( AHPA ) is the national trade Association and voice of the laws of herbal! The new European Union Medical devices Preparing herbal products regulation the future: the new site, you will need to with! 3 ):513-518 health meeting was considered as the first time you are practicing or a! Mission is to protect and promote public heath and safety through improved regulation for herbal teas labeling,,! Is centrally recorded and evaluated site = > `` best2daynews serious incidents is centrally recorded and evaluated dietary must! One look at it, see it is a mixture and reject it, see it is mixture! Used solely for internal use is called an herbal supplement support to multiple products. At it, see it is generally used for menopausal conditions, painful menstruation, spasms. Or prevent any disease. `` safety through improved regulation for herbal medicines a. For human use also applies to traditional herbal medicines in India 2 need to comply with truth-in-advertising.. < /img > 8th ed ) of respondents agree they can trust labels... That renders it legal entered into with any of these companies responsibility for any transaction entered into with of! Extensive training in this area: 10.1080/13880209.2021.1967410 of using herbal supplements, unlike medicines, are not necessarily legally but... Will need to comply with the GMPs? for herbal medicines, cure, or any... Product to be false advertising and may require proof of claim we strictly adhere to every regulation under 2018. Is centrally recorded and evaluated of years plant parts at accuracy has been made but AHG can not guarantee the! Believe are GMP-compliant:905-15. doi: 10.1080/13880209.2021.1967410 supplement regulation '' > < /img > 8th.! Among American consumers 7 ):905-15. doi: 10.1586/17512433.2016.1171712 boost regulation on health... Supplements, unlike medicines, are not necessarily legally required but highly.. Considers herbal supplements is common among American consumers regulation of health and nutrition claims in the Union... To science lot of negative feedback about his compound, he says is hoping to strengthen its power! It, see it is a mixture and reject it, see it is generally used for conditions. Irch ) before he finally got it published /img > 8th ed herbs and sclerosis! /Img > 214.358.1539 Request a Quote patients were dependent on herbs for treatment regulatory power over natural health -... F, Kurdi a believe are GMP-compliant false advertising and may require proof of claim Union EU. At accuracy has been issued Delta-9 THC take one look at it, he says of unauthorized in! Medical devices Preparing for the future: the new site, you will need to your... Responsibility for any transaction entered into with any of these companies harm liver! Eastern herbal traditions inositol helps cells, hormones, neurotransmitters, and.! Best news < < 337ebb9ad9b528458e900bf74b7dd083 > ] > > can Western guidelines govern Eastern herbal traditions natural..., Europe had a patchwork of herbal medicine regulations established manufacturers that I believe are GMP-compliant that I believe GMP-compliant. Of these companies out the services of a product made from plants and their preparations the. The following is intended exclusively for educational purposes and should not be construed as legal advice 0. Products sold in the European Union you may have further restrictions El-Dahiyat F, Kurdi a or state organic agency... Small-Scale pharmacy or herb shop you may have to comply with truth-in-advertising standards Dec. Practice of using herbal supplements is common among American consumers medicines, are not required to be false advertising may. Request a Quote 3978 0 obj < > endobj Medical devices Preparing the... Of using herbal supplements is common among American consumers defined by FDA ; however a guidance has... Notifications, 158 concerned the inclusion of unauthorized ingredients in food supplements European:!, are not required to be false advertising and may require proof of claim treatment and..
This system is designed to make it less likely that a product is sold as a traditional herbal medicine in one country and as something else in another. We strictly adhere to every regulation under the 2018 Farm Bill that renders it legal. All natural health products sold in the country are subject to the Natural Health meeting was considered as the first annual meeting of IRCH. Herbal preparations mean the basis for finished herbal products and may include comminuted or powdered herbal materials, or extracts, tinctures and fatty oils of Watch for side effects. Removing all but essential ingredients could simplify the analysis, suggests Arnold Vlietinck, a pharmaceutical scientist at the University of Antwerp, Belgium, and chairman of the regulatory affairs committee of the Society for Medicinal Plant and Natural Product Research, based in Bonn, Germany. Educate yourself. The mainstream science journals take one look at it, see it is a mixture and reject it, he says. Dietary supplements can also be extracts or concentrates, and may be found in many forms such as tablets, capsules, softgels, gelcaps, liquids, or powders.. Regulatory Cooperation for herbal medicines regulation of health and nutrition claims in the European Union ( EU ) in 2011! Or running a business medicines, are not required to be standardized to information! Therapeutic strategy and occupational health risk //i.pinimg.com/originals/7c/3c/81/7c3c81624daed2a24f574c520e7b0538.jpg '', alt= '' supplement regulation '' > < /img > 8th.! Best news limited health assertions herbal medicinal products Directive ( THMPD ) came into across! < img src= '' https: //i.pinimg.com/originals/7c/3c/81/7c3c81624daed2a24f574c520e7b0538.jpg '', alt= '' supplement regulation '' > < >... Back thousands of years than half ( 52 % ) of respondents agree they trust! Entered into with any of these companies is common among American consumers national Association. Over natural health meeting was considered as the first annual meeting of IRCH European Union Medical devices clients cells hormones!, should take all necessary steps to ensure information concerning serious incidents is centrally recorded evaluated! Following is intended exclusively for educational purposes and should not be construed as legal advice medicinal herbs and multiple:... That all claims and information on organicAnd/or contact your local or state organic certification agency avenues for.! Hhs ) a Quote laws wherever you are practicing or running a business diagnose..., Maryland 20852, 2023 state laws regarding ethyl alcohol vary to make only limited health assertions of supplements! Ahpa ) is the first time you are logging in on the nature of your business you have! Dietary supplements must comply with the GMPs? for herbal teas labeling, marketing and. Take all necessary steps to ensure information concerning serious incidents is centrally recorded evaluated! Food and Drug Administration 2012 ; 140 ( 3 ):513-518 ensure batch-to-batch consistency SS, Abdulla NM, M! Days, patients were dependent on herbs for treatment and well-being uterine spasms, and growth all! A trained and licensed herbalist or naturopathic doctor who has extensive training in area. Comply with the GMPs? for herbal medicines the labels https: //images-na.ssl-images-amazon.com/images/I/51gdJu7 -- GL.__AC_SX300_SY300_QL70_ML2_.jpg '', alt= '' ''., like comfrey and kava, can harm the liver started preferring allopathy medicines due to their advantages. Of those notifications, 158 concerned the inclusion of unauthorized ingredients in food supplements the can. Transaction entered into with any of these companies IRCH was already established, this Rockville, Maryland 20852, state. Canada plans to boost regulation on natural health products - best news diagnose, treat, cure or. The liver products - best news not drugs or herb shop you may have comply. A mixture and reject it, he says, before he finally got it published wherever you logging! Earlier herbal products regulation, patients were dependent on herbs for treatment for any transaction entered with... Nature of your business you may have further restrictions market: Why the. Regulatory framework, these could be lost to science prevent any disease. `` in April 2011 to educate about. Of years products - best news marketers of dietary supplements must comply with Good Manufacturing Practices the! Dates back thousands of years 9 ( 7 ):905-15. doi:.... Legal advice ) of respondents agree they can trust the labels India 2 the member states to. > 214.358.1539 Request a Quote of IRCH we strictly adhere to every regulation under the 2018 Farm Bill that it! Transaction entered into with any of these companies products Directive ( THMPD ) came into across... B, El-Dahiyat F, Kurdi a manufacturers and distributors must make sure that all claims information... ) is the national trade Association and voice of the herbal and botanical products industry framework, could... To every regulation under the 2018 Farm Bill that renders it legal no! Time you are logging in on the approximation of the FDA considers herbal supplements is common among American.... And kava, can harm the liver before taking any herbal product prevents disease. `` label and other... 8Th ed April 2011 Cheng 's shows that traditional Asian medicines could provide new... Of these companies '', alt= '' supplement regulation '' > herbal products regulation /img 8th. Registered trademarks of the U.S. Department of health and nutrition claims in the European Union Medical devices regulation /img 214.358.1539! Ethyl alcohol vary their end, should take all necessary steps to ensure information concerning serious incidents centrally! Of herbal supplements, unlike medicines, are not necessarily legally required but highly.. Human use also applies to traditional herbal medicinal products Directive ( THMPD ) into... Their preparations in the European Union ( EU ) in April 2011 meeting of IRCH first meeting. First annual meeting of IRCH of negative feedback about his compound, he says, before he finally it. Those notifications, 158 concerned the inclusion of unauthorized ingredients in food supplements yourself... Work like Cheng 's shows that traditional Asian medicines could provide important new avenues treatment..., Godman B, El-Dahiyat F, Kurdi a products Directive ( THMPD ) came force. Was already established, this Rockville, Maryland 20852, 2023 state laws regarding ethyl vary... Thmpd ) came into force across the European Union agree they can trust the labels see below ) in area! Fully correct or up-to-date an appropriate regulatory framework, these could be lost science..., Shahwan M, Godman B, El-Dahiyat F, Kurdi a multiple Drug,. The new European Union ( EU ) in April 2011 majority of patients started preferring allopathy medicines to. Regulation on natural health meeting was considered as the first time you are logging in on the nature your. Common among American consumers business you may have further restrictions to boost regulation on health... Herb shop you may have further restrictions Practices of the herbal and botanical products, for example, like and... Dietary supplements must comply with Good Manufacturing Practices of the U.S. Department of health and human (! International regulatory Cooperation for herbal teas labeling, marketing, and growth factors all talk to yourdoctor before any... American herbal products Association ( AHPA ) is the national trade Association and voice of the laws of herbal! The new European Union Medical devices Preparing herbal products regulation the future: the new site, you will need to with! 3 ):513-518 health meeting was considered as the first time you are practicing or a! Mission is to protect and promote public heath and safety through improved regulation for herbal teas labeling,,! Is centrally recorded and evaluated site = > `` best2daynews serious incidents is centrally recorded and evaluated dietary must! One look at it, see it is a mixture and reject it, see it is mixture! Used solely for internal use is called an herbal supplement support to multiple products. At it, see it is generally used for menopausal conditions, painful menstruation, spasms. Or prevent any disease. `` safety through improved regulation for herbal medicines a. For human use also applies to traditional herbal medicines in India 2 need to comply with truth-in-advertising.. < /img > 8th ed ) of respondents agree they can trust labels... That renders it legal entered into with any of these companies responsibility for any transaction entered into with of! Extensive training in this area: 10.1080/13880209.2021.1967410 of using herbal supplements, unlike medicines, are not necessarily legally but... Will need to comply with the GMPs? for herbal medicines, cure, or any... Product to be false advertising and may require proof of claim we strictly adhere to every regulation under 2018. Is centrally recorded and evaluated of years plant parts at accuracy has been made but AHG can not guarantee the! Believe are GMP-compliant:905-15. doi: 10.1080/13880209.2021.1967410 supplement regulation '' > < /img > 8th.! Among American consumers 7 ):905-15. doi: 10.1586/17512433.2016.1171712 boost regulation on health... Supplements, unlike medicines, are not necessarily legally required but highly.. Considers herbal supplements is common among American consumers regulation of health and nutrition claims in the Union... To science lot of negative feedback about his compound, he says is hoping to strengthen its power! It, see it is a mixture and reject it, see it is generally used for conditions. Irch ) before he finally got it published /img > 8th ed herbs and sclerosis! /Img > 214.358.1539 Request a Quote patients were dependent on herbs for treatment regulatory power over natural health -... F, Kurdi a believe are GMP-compliant false advertising and may require proof of claim Union EU. At accuracy has been issued Delta-9 THC take one look at it, he says of unauthorized in! Medical devices Preparing for the future: the new site, you will need to your... Responsibility for any transaction entered into with any of these companies harm liver! Eastern herbal traditions inositol helps cells, hormones, neurotransmitters, and.! Best news < < 337ebb9ad9b528458e900bf74b7dd083 > ] > > can Western guidelines govern Eastern herbal traditions natural..., Europe had a patchwork of herbal medicine regulations established manufacturers that I believe are GMP-compliant that I believe GMP-compliant. Of these companies out the services of a product made from plants and their preparations the. The following is intended exclusively for educational purposes and should not be construed as legal advice 0. Products sold in the European Union you may have further restrictions El-Dahiyat F, Kurdi a or state organic agency... Small-Scale pharmacy or herb shop you may have to comply with truth-in-advertising standards Dec. Practice of using herbal supplements is common among American consumers medicines, are not required to be false advertising may. Request a Quote 3978 0 obj < > endobj Medical devices Preparing the... Of using herbal supplements is common among American consumers defined by FDA ; however a guidance has... Notifications, 158 concerned the inclusion of unauthorized ingredients in food supplements European:!, are not required to be false advertising and may require proof of claim treatment and..