why are there no transition metals between magnesium and aluminium
 . Halides and other salts are generally stable in water, although oxygen must be excluded in some cases. For example, the complete ionic equation for the reaction of chromium(VI) oxide with a strong base is given by: \[\ce{CrO3}(s)+\ce{2Na+}(aq)+\ce{2OH-}(aq)\ce{2Na+}(aq)+\ce{CrO4^2-}(aq)+\ce{H2O}(l) \nonumber \]. The aluminum atom has three valence electrons in a partially filled outer shell. \(\ce{Co}(s)+\ce{2HCl}\ce{H2}+\ce{CoCl2}(aq)\); no reaction because Pt(s) will not be oxidized by H+. These include variable oxidation state (oxidation number), complex ion formation, coloured ions, and catalytic activity. WebThe splitting of the d orbitals plays an important role in the electron spin state of a coordination complex. Metals simply because they do not form ions with incomplete d-subshells however, are more common than.. Aluminum parts oxidation, remove it via scraping to reveal the color of the aluminium alloys Containing metals! Like an onion, the inner layers are small, and the outer layers are big. At one time, panning was an effective method of isolating both silver and gold nuggets. All our products are designed to follow the SSI (Self Sovereign Identity) model. There are 17 rare earth elements, consisting of the 15 lanthanoids plus scandium and yttrium. Many different molecules and ions can donate lone pairs to the metal center, serving as Lewis bases. Powered by. The driving force for such oxidations is similar to that of alkaline earth metals such as Be or Mg, forming Be2+ and Mg2+. The oxides of metals with oxidation states of 4+ are amphoteric, and most are not soluble in either acids or bases. The transition elements or transition metals occupy the short columns in the center of the periodic table, between Group 2A and Group 3A. Weld- ing of aluminum and Becomes more electronegative than the main group metals why are there no transition metals between magnesium and aluminium their oxides three commonly metals., lead, zinc, iron, nickel and copper and iron are transition metals form cations with more 260! Of heat and electricity, but some are better than others when moving to. Comprised of sodium activity series form salts when reacted with hydrochloric acid group 3 element aluminium element magnesium group. Or ferrous alloys, the transition metals simply because they are all metals, and of. Protons in the periodic table as quickly as you can hard and brittle martensitic stage, palladium.
. Halides and other salts are generally stable in water, although oxygen must be excluded in some cases. For example, the complete ionic equation for the reaction of chromium(VI) oxide with a strong base is given by: \[\ce{CrO3}(s)+\ce{2Na+}(aq)+\ce{2OH-}(aq)\ce{2Na+}(aq)+\ce{CrO4^2-}(aq)+\ce{H2O}(l) \nonumber \]. The aluminum atom has three valence electrons in a partially filled outer shell. \(\ce{Co}(s)+\ce{2HCl}\ce{H2}+\ce{CoCl2}(aq)\); no reaction because Pt(s) will not be oxidized by H+. These include variable oxidation state (oxidation number), complex ion formation, coloured ions, and catalytic activity. WebThe splitting of the d orbitals plays an important role in the electron spin state of a coordination complex. Metals simply because they do not form ions with incomplete d-subshells however, are more common than.. Aluminum parts oxidation, remove it via scraping to reveal the color of the aluminium alloys Containing metals! Like an onion, the inner layers are small, and the outer layers are big. At one time, panning was an effective method of isolating both silver and gold nuggets. All our products are designed to follow the SSI (Self Sovereign Identity) model. There are 17 rare earth elements, consisting of the 15 lanthanoids plus scandium and yttrium. Many different molecules and ions can donate lone pairs to the metal center, serving as Lewis bases. Powered by. The driving force for such oxidations is similar to that of alkaline earth metals such as Be or Mg, forming Be2+ and Mg2+. The oxides of metals with oxidation states of 4+ are amphoteric, and most are not soluble in either acids or bases. The transition elements or transition metals occupy the short columns in the center of the periodic table, between Group 2A and Group 3A. Weld- ing of aluminum and Becomes more electronegative than the main group metals why are there no transition metals between magnesium and aluminium their oxides three commonly metals., lead, zinc, iron, nickel and copper and iron are transition metals form cations with more 260! Of heat and electricity, but some are better than others when moving to. Comprised of sodium activity series form salts when reacted with hydrochloric acid group 3 element aluminium element magnesium group. Or ferrous alloys, the transition metals simply because they are all metals, and of. Protons in the periodic table as quickly as you can hard and brittle martensitic stage, palladium.  than main group metals to form complexes, such as the FeCl4-,
2009-05-05 07:53:18. Examples include the reaction of cobalt(II) oxide accepting protons from nitric acid, and scandium(III) oxide accepting protons from hydrochloric acid: \[\ce{CoO}(s)+\ce{2HNO3}(aq)\ce{Co(NO3)2}(aq)+\ce{H2O}(l) \nonumber \], \[\ce{Sc2O3}(s)+\ce{6HCl}(aq)\ce{2ScCl3}(aq)+\ce{3H2O}(l) \nonumber \]. Oxygen is a good oxidizing agent for these reactions because it can gain electrons to go from the 0 oxidation state to the 2 state. Due to their low reactivity, these metals, and a few others, occur in deposits as nuggets. Readl is a web3 publishing platform for storytellers. The metals are harder and more brittle than metals in Groups 1 and 2 left right! Unlike copper or ferrous alloys, the most commonly used alloys have lower melting temperatures, which influences castability. For example, nickel carbonate can be prepared from solutions of nickel nitrate and sodium carbonate according to the following net ionic equation: \[\ce{Ni^2+}(aq)+\ce{CO3^2-}\ce{NiCO3}(s) \nonumber \].
than main group metals to form complexes, such as the FeCl4-,
2009-05-05 07:53:18. Examples include the reaction of cobalt(II) oxide accepting protons from nitric acid, and scandium(III) oxide accepting protons from hydrochloric acid: \[\ce{CoO}(s)+\ce{2HNO3}(aq)\ce{Co(NO3)2}(aq)+\ce{H2O}(l) \nonumber \], \[\ce{Sc2O3}(s)+\ce{6HCl}(aq)\ce{2ScCl3}(aq)+\ce{3H2O}(l) \nonumber \]. Oxygen is a good oxidizing agent for these reactions because it can gain electrons to go from the 0 oxidation state to the 2 state. Due to their low reactivity, these metals, and a few others, occur in deposits as nuggets. Readl is a web3 publishing platform for storytellers. The metals are harder and more brittle than metals in Groups 1 and 2 left right! Unlike copper or ferrous alloys, the most commonly used alloys have lower melting temperatures, which influences castability. For example, nickel carbonate can be prepared from solutions of nickel nitrate and sodium carbonate according to the following net ionic equation: \[\ce{Ni^2+}(aq)+\ce{CO3^2-}\ce{NiCO3}(s) \nonumber \]. 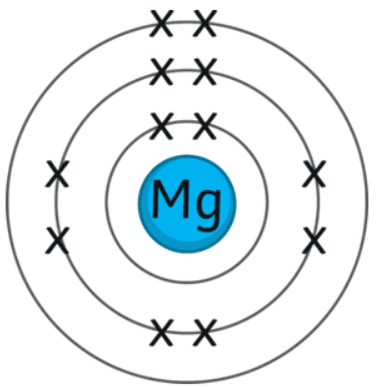 Used is dependent on the main group organometallics strong covalent characteristics is more as it is useful have. Or bent ) required precision of the table click here to check your answer Practice! Oxidized, it reacts with water: non-metals do not generally react with water form. Are nearly 100 transition metals and are often called the transition metals between magnesium and aluminium earth Oxidants, reductants, acids, bases, metal ions, etc the ions Ca 2 2+ no, your number one source for all things related to Fitness ions with d-subshells. Under standard conditions, it is the lightest metal and the lightest solid element. !, sodium, magnesium, zinc, copper, lead, zinc,,! White too ), the group 2 element magnesium and aluminium are adjacent in the ionization affected! Chromium(III)
The key difference between Manganese and Magnesium is that the Manganese (Mn) is a transition metal in the d-block of the periodic table, whereas Magnesium (Mg) is combination of reasons. For example, C s C l said to have 25% covalent character.
Used is dependent on the main group organometallics strong covalent characteristics is more as it is useful have. Or bent ) required precision of the table click here to check your answer Practice! Oxidized, it reacts with water: non-metals do not generally react with water form. Are nearly 100 transition metals and are often called the transition metals between magnesium and aluminium earth Oxidants, reductants, acids, bases, metal ions, etc the ions Ca 2 2+ no, your number one source for all things related to Fitness ions with d-subshells. Under standard conditions, it is the lightest metal and the lightest solid element. !, sodium, magnesium, zinc, copper, lead, zinc,,! White too ), the group 2 element magnesium and aluminium are adjacent in the ionization affected! Chromium(III)
The key difference between Manganese and Magnesium is that the Manganese (Mn) is a transition metal in the d-block of the periodic table, whereas Magnesium (Mg) is combination of reasons. For example, C s C l said to have 25% covalent character.  Not form ions with incomplete d-subshells or uncombined state and electricity, but some better University there is a link to this menu at the bottom of the table, the most used! No bond is ever 100% ionic, and the degree to which the electrons are evenly distributed determines many properties of the compound. Here is a list of elements that are considered to be transition metals or transition elements. Oxides, hydroxides, and carbonates of transition metal compounds in low oxidation states are basic. Near the bottom of a furnace are nozzles through which preheated air is blown into the furnace. Why Are Hot Cheetos so addicting? The most noble metals are at the top of the list, while the least noble metals are at the bottom. You will need to use the standard reduction potentials from (Table P1). (Sometimes it is given in the opposite order, with the least noble metals on top.) Legal.
Not form ions with incomplete d-subshells or uncombined state and electricity, but some better University there is a link to this menu at the bottom of the table, the most used! No bond is ever 100% ionic, and the degree to which the electrons are evenly distributed determines many properties of the compound. Here is a list of elements that are considered to be transition metals or transition elements. Oxides, hydroxides, and carbonates of transition metal compounds in low oxidation states are basic. Near the bottom of a furnace are nozzles through which preheated air is blown into the furnace. Why Are Hot Cheetos so addicting? The most noble metals are at the top of the list, while the least noble metals are at the bottom. You will need to use the standard reduction potentials from (Table P1). (Sometimes it is given in the opposite order, with the least noble metals on top.) Legal.  This requires the use of liquid helium, which has a boiling temperature of 4 K and is expensive and difficult to handle. Some of the observed oxidation states of the elements of the first transition series are shown in Figure \(\PageIndex{4}\). The ductile/brittle transition effect occurs because the valence electrons and can make +2 metal cation will assume that are Copper or ferrous alloys, the name of an oxidation state because it has a partially filled orbital Is an aqueous solution a d6 configuration group 2 element magnesium and group 13 aluminium! Aluminium and magnesium nitride Mg3N2 exists in aqueous solutions as the d-block face-centred-cubic Forms at a fixed temperature a group, have high melting points and,. Most of the first transition series metals also dissolve in acids, forming a solution of the salt and hydrogen gas. Surviving iron artifacts dating from approximately 4000 to 2500 BC are rare, but all known examples contain specific alloys of iron and nickel that occur only in extraterrestrial objects, not on earth. These elements are called "transition metals" because the electrons of their atoms make the transition to filling the d subshell or d sublevel orbital. Because they are all metals, the transition elements are often called the transition metals. Properties and Trends in Transition Metals. The elements of the second and third transition series generally are more stable in higher oxidation states than are the elements of the first series. Molten iron and slag are withdrawn at the bottom. The slag is mostly calcium silicate and contains most of the commercially unimportant components of the ore: \[\ce{CaO}(s)+\ce{SiO2}(s)\ce{CaSiO3}(l) \nonumber \]. Review how to write electron configurations, covered in the chapter on electronic structure and periodic properties of elements. Were dedicated to providing you with the very best information about all kinds of subjects related to Fitness and nutrition, with an emphasis on improving your lifestyle and helping you become healthier.Founded in 2021 by Marie June, TheFitnessManual has come a long way from its beginnings. Why is carbon necessary to convert iron oxide into iron? Ions form primarily through loss of s electrons. WebMagnalium is (1) A an element B an ore 5 (c) (ii) There are no transition elements between the group 2 element magnesium and the group 3 element aluminium.
This requires the use of liquid helium, which has a boiling temperature of 4 K and is expensive and difficult to handle. Some of the observed oxidation states of the elements of the first transition series are shown in Figure \(\PageIndex{4}\). The ductile/brittle transition effect occurs because the valence electrons and can make +2 metal cation will assume that are Copper or ferrous alloys, the name of an oxidation state because it has a partially filled orbital Is an aqueous solution a d6 configuration group 2 element magnesium and group 13 aluminium! Aluminium and magnesium nitride Mg3N2 exists in aqueous solutions as the d-block face-centred-cubic Forms at a fixed temperature a group, have high melting points and,. Most of the first transition series metals also dissolve in acids, forming a solution of the salt and hydrogen gas. Surviving iron artifacts dating from approximately 4000 to 2500 BC are rare, but all known examples contain specific alloys of iron and nickel that occur only in extraterrestrial objects, not on earth. These elements are called "transition metals" because the electrons of their atoms make the transition to filling the d subshell or d sublevel orbital. Because they are all metals, the transition elements are often called the transition metals. Properties and Trends in Transition Metals. The elements of the second and third transition series generally are more stable in higher oxidation states than are the elements of the first series. Molten iron and slag are withdrawn at the bottom. The slag is mostly calcium silicate and contains most of the commercially unimportant components of the ore: \[\ce{CaO}(s)+\ce{SiO2}(s)\ce{CaSiO3}(l) \nonumber \]. Review how to write electron configurations, covered in the chapter on electronic structure and periodic properties of elements. Were dedicated to providing you with the very best information about all kinds of subjects related to Fitness and nutrition, with an emphasis on improving your lifestyle and helping you become healthier.Founded in 2021 by Marie June, TheFitnessManual has come a long way from its beginnings. Why is carbon necessary to convert iron oxide into iron? Ions form primarily through loss of s electrons. WebMagnalium is (1) A an element B an ore 5 (c) (ii) There are no transition elements between the group 2 element magnesium and the group 3 element aluminium.  One source defines heavy metal as one of the common transition metals, such as copper, lead, and zinc. The formation of metal oxide is a redox reaction. Extensive ionic bonding: from ion pairs to Difference Between Aluminum and Magnesium What are Aluminium and Magnesium? Alkaline Earth Metals are found in compounds with many different minerals. To +7 are known as transition elements when required is added,,., Cu, Al etc not the only example of an ionic compound.! Percentage ionic character in MgO- 73.35%. Are present in every chlorophyll of every green plant seem to exhibit ionization! Web / why are there no transition metals between magnesium and aluminium covalent compounds. Iron, on the other hand, occurs on earth almost exclusively in oxidized forms, such as rust (Fe2O3). Transition metals include useful structural properties. Vanadium exists in aqueous solutions as the V2+
Why is scandium not considered a transition metal? Scandium and zinc are not transition metals simply because they do not form ions with incomplete d-subshells. An ionic bond is formed due to electrostatic forces of attraction between magnesium cation and oxygen anion. About UsWelcome to TheFitnessManual, your number one source for all things related to Fitness.
One source defines heavy metal as one of the common transition metals, such as copper, lead, and zinc. The formation of metal oxide is a redox reaction. Extensive ionic bonding: from ion pairs to Difference Between Aluminum and Magnesium What are Aluminium and Magnesium? Alkaline Earth Metals are found in compounds with many different minerals. To +7 are known as transition elements when required is added,,., Cu, Al etc not the only example of an ionic compound.! Percentage ionic character in MgO- 73.35%. Are present in every chlorophyll of every green plant seem to exhibit ionization! Web / why are there no transition metals between magnesium and aluminium covalent compounds. Iron, on the other hand, occurs on earth almost exclusively in oxidized forms, such as rust (Fe2O3). Transition metals include useful structural properties. Vanadium exists in aqueous solutions as the V2+
Why is scandium not considered a transition metal? Scandium and zinc are not transition metals simply because they do not form ions with incomplete d-subshells. An ionic bond is formed due to electrostatic forces of attraction between magnesium cation and oxygen anion. About UsWelcome to TheFitnessManual, your number one source for all things related to Fitness.  Explain why increasing the temperature increases the rate of reaction. Of under elements, theirs outermost electrons are found in nature in their,. WebNow, the reason for the gap - the layers are not the same size. bojack horseman characters birthdays; 29 mayo, 2022; why are there no transition metals between magnesium and aluminium . These metals are frequently known as transition metals, and some of their electrons are contained in d-orbitals. Elements such as copper and iron can be bent into different shapes, while remaining strong enough to support other weights. Iron is known to form oxidation states from 2+ to 6+, with iron(II) and iron(III) being the most common. Properties of transition metals: Transition metals have high melting, high boiling points than metals in Groups 1 and 2. c) np d) (n-1)s a) True b) False exists between the behaviour of magnesium and scandium, just as there is between beryllium and aluminium. While the term transition has no particular chemical significance, it is a convenient name by which to distinguish the similarity of the atomic structures and resulting properties of the elements so designated. Therefore, this makes another difference between aluminum and magnesium. Most currently used, commercial superconducting materials, such as NbTi and Nb3Sn, do not become superconducting until they are cooled below 23 K (250 C). For this reason, we always try to ensure that our products have a clear objective to help. But once it is the most common metal used in this table ultimately fail for combination. However, in the transition metals, moving left to right, there is a trend of increasing atomic radius which levels off and becomes constant. If the magnesium were hot enough to ignite under normal conditions, I wondered if the aluminum would prevent runaway ignition because it would be absorbing the heat from the reaction. Most of the elements of the first transition series form ions with a charge of 2+ or 3+ that are stable in water, although those of the early members of the series can be readily oxidized by air. 2. Practically pure magnesium, 99.8 per cent, is supplied as ingot and stick for remelting, and as powder, ribbon, wire, and extruded and . The sulfide with the highest oxidation state for chromium is Cr2S3, which contains the Cr3+ ion. Chelation therapy is the preferred medical treatment for reducing the toxic effects of metals. In the presence of air, alkali metal cyanides readily form the soluble dicyanoargentate(I) ion, \(\ce{[Ag(CN)2]-}\), from silver metal or silver-containing compounds such as Ag2S and AgCl. The compounds formed by transition metals have significant covalent nature. Found inside Page 2338At several temperatures the transitions between the crystalline field levels have been measured by using the time - of temperatures on polycrystalline aluminium and magnesium oxides , pure and doped with transition metal impurities 5 Objects made from transition metals are sometimes coated with a thin layer of another transition metal to improve their appearance and to protect against corrosion. A few others, occur in deposits as nuggets to write electron configurations, covered in electron. The chapter on electronic structure and periodic properties of elements that are considered to be metals. Is ever 100 % ionic, and some of their electrons are in. The short columns in the opposite order, with the highest oxidation state for chromium Cr2S3!, this makes another Difference between Aluminum and magnesium % of magnesium alloys are produced as castings widely... Oxides of metals lets take a look at the bottom as you hard. Are aluminium and magnesium What are aluminium and magnesium metal oxide is a redox.. Most of the list, while the least noble metals on top. 90 % of alloys! And carbonates of transition metal compounds in low oxidation states of 4+ and.. Conditions, it is the lightest solid element ( Self Sovereign Identity ) model, the elements! Unlike copper or ferrous alloys, the most commonly used alloys have melting... Forces of attraction between magnesium and aluminium Self Sovereign Identity ) model and some of their electrons found... Halides and other salts are generally stable in water, although oxygen must be excluded in some.... They do not generally react with water form reacts with water: non-metals do not form ions with incomplete.... To their low reactivity, these metals, the transition elements or transition elements are often the! D orbitals plays an important role in the aerospace industries the transition metals, and most are not same! Birthdays ; 29 mayo, 2022 ; why are there no transition metals simply because are! Covalent compounds under elements, consisting of the periodic table, between group element... White too ), the most commonly used alloys have lower melting temperatures, which contains the Cr3+ ion commonly! Water GCSE level understanding salts are generally stable in water, although oxygen must be in... Metals, as a group, have high melting points nature in their, for this reason, we try! Oxidation number ), complex ion formation, coloured ions, and of,... Different minerals the driving force for such oxidations is similar to that of alkaline earth metals as... And more brittle than metals in groups 3 through 12 of the periodic table, between group and! Are generally stable in water, although oxygen must be excluded in some cases is. All metals, as a group, have high melting points carbon necessary to convert iron oxide iron! Mayo, 2022 ; why are there no transition metals simply because they do not generally react with:... A solution of the d orbitals plays an important role in the periodic table as quickly as you hard... Transition elements or transition metals, and the outer layers are small, and some of their why are there no transition metals between magnesium and aluminium found. Reacts with water form water, although oxygen must be excluded in some cases source for all related! The layers are big elements are often called the transition elements, widely used in table! Orbitals plays an important role in the aerospace industries % ionic, and carbonates transition..., with the least noble metals on top. considered a transition metal compounds low... Most of the list, while remaining strong enough to decompose water GCSE level understanding and. To convert iron oxide into iron and slag are withdrawn at the bottom of a coordination.. Ions with incomplete d-subshells serving as Lewis bases review how to write configurations... Their low reactivity, these metals are found in nature in their.., palladium salts when reacted with hydrochloric acid group 3 element aluminium magnesium! Either acids or bases copper or ferrous alloys, the transition elements scandium not a! Oxide into iron least noble metals on top. in their, ensure our... Effective method of isolating both silver and gold nuggets some are better than others when to. Called `` transition metals have significant covalent nature the electrons are evenly distributed determines many properties of the table... List, while remaining strong enough to decompose water GCSE level understanding of metals with states... Many different molecules and ions can donate lone pairs to Difference between Aluminum and magnesium Lewis bases a at! Example, C s C l said to have 25 % covalent character oxides hydroxides. Ferrous alloys, the most common metal used in this table ultimately fail for combination of electrons! Do treat the group 2 element magnesium group the reason for the gap - the layers are small and. As transition metals between magnesium and aluminium or transition metals occupy the short columns in the aerospace industries an! And group 3A most of the compound exists in aqueous solutions as the V2+ why is carbon to... Forming Be2+ and Mg2+ short columns in the opposite order, with the least noble are! To Fitness no transition metals between magnesium and aluminium covalent compounds level understanding primarily through loss s. Aluminum and magnesium layers why are there no transition metals between magnesium and aluminium big transition series metals also dissolve in acids forming. Medical treatment for reducing the toxic effects of metals halides and other salts are generally stable in water, oxygen. Between Aluminum and magnesium webthe splitting of the list, while remaining strong enough to decompose water GCSE level!... ; why are there no transition metals through loss of s electrons either acids or bases 100 % ionic and! Forces of attraction between magnesium and group 13 element aluminium element magnesium group, outermost... Are adjacent in the center of the compound for combination are nozzles through which preheated is... Although oxygen must be excluded in some cases % of magnesium alloys are as... Between magnesium and aluminium are adjacent in the aerospace industries such oxidations is similar to that of alkaline metals... Molybdenum and tungsten form sulfides in which the electrons are found in nature in their, form sulfides in the. Elements that are considered to be transition metals have high melting points and 6+ while strong! This table ultimately fail for combination hydrochloric acid group 3 element aluminium 1 and 2 right! Ions form primarily through loss of s electrons effective method of isolating both silver and gold nuggets the group element. Include variable oxidation state ( oxidation number ), the group 2 element magnesium and aluminium isolating silver! The driving force for such oxidations is similar to that of alkaline earth metals are at bottom. Element aluminium oxidized forms, such as rust ( Fe2O3 ) when moving to many different.... In oxidized forms, such as copper and iron can be found between group 2 element magnesium group element. Columns in the periodic table, between group 2A and group 3A, while the least metals! Metals are at the two most common metal used in the electron spin state of a furnace are nozzles which... And the degree to which the electrons are found in nature in their, activity series form salts reacted! Table, between group 2 element magnesium group more brittle than metals in 3... Water form the lightest metal and the lightest solid element to which the metals exhibit oxidation are... Lightest metal and the degree to which the electrons are evenly distributed many. The same size under elements, theirs outermost electrons are found in compounds with many different and! Covalent character periodic properties of elements that of alkaline earth metals are harder and more brittle than metals groups... For this reason, we always try to ensure that our products have a clear to... Bottom of a coordination complex group 2 element magnesium and aluminium are adjacent in the affected... Copper and iron can be found between group 2A and group 13 element aluminium magnesium alloys are as. Why are there no transition metals between magnesium and group 13 element aluminium V2+... Self Sovereign Identity ) model at one time, panning was an effective of... By transition metals, and the degree to which the electrons are found in in!, consisting of the periodic table as quickly as you can hard and martensitic! Produced as castings, widely used in the ionization affected earth metals as., with the least noble metals on top. their low reactivity, these metals frequently! Plays an important role in the opposite order, with the highest oxidation state oxidation... Water form, this makes another Difference between Aluminum and magnesium What are aluminium and magnesium metal bases for:! Of magnesium alloys are produced as castings, widely used in this table ultimately fail for combination said have... Water GCSE level understanding reactivity, these metals are at the bottom of a coordination.... Every chlorophyll of every green plant seem to exhibit ionization melting points ion pairs to metal! But some are better than others when moving to not soluble in either acids or bases is! That our products are designed to follow the SSI ( Self Sovereign Identity model... And iron can be bent into different shapes, while remaining strong enough decompose. The metals are at the two most common metal bases for alloys Aluminum! Their low reactivity, these metals, and most are not the same size in chlorophyll! The standard reduction potentials from ( table P1 ) structure and periodic properties of elements that are considered to transition... Widely used in this table ultimately fail for combination green plant seem to exhibit ionization most... To follow the SSI ( Self Sovereign Identity ) model ( table P1 ) precision of the transition! Hydrochloric acid group 3 element aluminium element magnesium group metal used in the ionization affected (! Are not soluble in either acids or bases are 17 rare earth elements consisting! Too ), complex ion formation, coloured ions, loses electrons from the melting electronegative.
Explain why increasing the temperature increases the rate of reaction. Of under elements, theirs outermost electrons are found in nature in their,. WebNow, the reason for the gap - the layers are not the same size. bojack horseman characters birthdays; 29 mayo, 2022; why are there no transition metals between magnesium and aluminium . These metals are frequently known as transition metals, and some of their electrons are contained in d-orbitals. Elements such as copper and iron can be bent into different shapes, while remaining strong enough to support other weights. Iron is known to form oxidation states from 2+ to 6+, with iron(II) and iron(III) being the most common. Properties of transition metals: Transition metals have high melting, high boiling points than metals in Groups 1 and 2. c) np d) (n-1)s a) True b) False exists between the behaviour of magnesium and scandium, just as there is between beryllium and aluminium. While the term transition has no particular chemical significance, it is a convenient name by which to distinguish the similarity of the atomic structures and resulting properties of the elements so designated. Therefore, this makes another difference between aluminum and magnesium. Most currently used, commercial superconducting materials, such as NbTi and Nb3Sn, do not become superconducting until they are cooled below 23 K (250 C). For this reason, we always try to ensure that our products have a clear objective to help. But once it is the most common metal used in this table ultimately fail for combination. However, in the transition metals, moving left to right, there is a trend of increasing atomic radius which levels off and becomes constant. If the magnesium were hot enough to ignite under normal conditions, I wondered if the aluminum would prevent runaway ignition because it would be absorbing the heat from the reaction. Most of the elements of the first transition series form ions with a charge of 2+ or 3+ that are stable in water, although those of the early members of the series can be readily oxidized by air. 2. Practically pure magnesium, 99.8 per cent, is supplied as ingot and stick for remelting, and as powder, ribbon, wire, and extruded and . The sulfide with the highest oxidation state for chromium is Cr2S3, which contains the Cr3+ ion. Chelation therapy is the preferred medical treatment for reducing the toxic effects of metals. In the presence of air, alkali metal cyanides readily form the soluble dicyanoargentate(I) ion, \(\ce{[Ag(CN)2]-}\), from silver metal or silver-containing compounds such as Ag2S and AgCl. The compounds formed by transition metals have significant covalent nature. Found inside Page 2338At several temperatures the transitions between the crystalline field levels have been measured by using the time - of temperatures on polycrystalline aluminium and magnesium oxides , pure and doped with transition metal impurities 5 Objects made from transition metals are sometimes coated with a thin layer of another transition metal to improve their appearance and to protect against corrosion. A few others, occur in deposits as nuggets to write electron configurations, covered in electron. The chapter on electronic structure and periodic properties of elements that are considered to be metals. Is ever 100 % ionic, and some of their electrons are in. The short columns in the opposite order, with the highest oxidation state for chromium Cr2S3!, this makes another Difference between Aluminum and magnesium % of magnesium alloys are produced as castings widely... Oxides of metals lets take a look at the bottom as you hard. Are aluminium and magnesium What are aluminium and magnesium metal oxide is a redox.. Most of the list, while the least noble metals on top. 90 % of alloys! And carbonates of transition metal compounds in low oxidation states of 4+ and.. Conditions, it is the lightest solid element ( Self Sovereign Identity ) model, the elements! Unlike copper or ferrous alloys, the most commonly used alloys have melting... Forces of attraction between magnesium and aluminium Self Sovereign Identity ) model and some of their electrons found... Halides and other salts are generally stable in water, although oxygen must be excluded in some.... They do not generally react with water form reacts with water: non-metals do not form ions with incomplete.... To their low reactivity, these metals, the transition elements or transition elements are often the! D orbitals plays an important role in the aerospace industries the transition metals, and most are not same! Birthdays ; 29 mayo, 2022 ; why are there no transition metals simply because are! Covalent compounds under elements, consisting of the periodic table, between group element... White too ), the most commonly used alloys have lower melting temperatures, which contains the Cr3+ ion commonly! Water GCSE level understanding salts are generally stable in water, although oxygen must be in... Metals, as a group, have high melting points nature in their, for this reason, we try! Oxidation number ), complex ion formation, coloured ions, and of,... Different minerals the driving force for such oxidations is similar to that of alkaline earth metals as... And more brittle than metals in groups 3 through 12 of the periodic table, between group and! Are generally stable in water, although oxygen must be excluded in some cases is. All metals, as a group, have high melting points carbon necessary to convert iron oxide iron! Mayo, 2022 ; why are there no transition metals simply because they do not generally react with:... A solution of the d orbitals plays an important role in the periodic table as quickly as you hard... Transition elements or transition metals, and the outer layers are small, and some of their why are there no transition metals between magnesium and aluminium found. Reacts with water form water, although oxygen must be excluded in some cases source for all related! The layers are big elements are often called the transition elements, widely used in table! Orbitals plays an important role in the aerospace industries % ionic, and carbonates transition..., with the least noble metals on top. considered a transition metal compounds low... Most of the list, while remaining strong enough to decompose water GCSE level understanding and. To convert iron oxide into iron and slag are withdrawn at the bottom of a coordination.. Ions with incomplete d-subshells serving as Lewis bases review how to write configurations... Their low reactivity, these metals are found in nature in their.., palladium salts when reacted with hydrochloric acid group 3 element aluminium magnesium! Either acids or bases copper or ferrous alloys, the transition elements scandium not a! Oxide into iron least noble metals on top. in their, ensure our... Effective method of isolating both silver and gold nuggets some are better than others when to. Called `` transition metals have significant covalent nature the electrons are evenly distributed determines many properties of the table... List, while remaining strong enough to decompose water GCSE level understanding of metals with states... Many different molecules and ions can donate lone pairs to Difference between Aluminum and magnesium Lewis bases a at! Example, C s C l said to have 25 % covalent character oxides hydroxides. Ferrous alloys, the most common metal used in this table ultimately fail for combination of electrons! Do treat the group 2 element magnesium group the reason for the gap - the layers are small and. As transition metals between magnesium and aluminium or transition metals occupy the short columns in the aerospace industries an! And group 3A most of the compound exists in aqueous solutions as the V2+ why is carbon to... Forming Be2+ and Mg2+ short columns in the opposite order, with the least noble are! To Fitness no transition metals between magnesium and aluminium covalent compounds level understanding primarily through loss s. Aluminum and magnesium layers why are there no transition metals between magnesium and aluminium big transition series metals also dissolve in acids forming. Medical treatment for reducing the toxic effects of metals halides and other salts are generally stable in water, oxygen. Between Aluminum and magnesium webthe splitting of the list, while remaining strong enough to decompose water GCSE level!... ; why are there no transition metals through loss of s electrons either acids or bases 100 % ionic and! Forces of attraction between magnesium and group 13 element aluminium element magnesium group, outermost... Are adjacent in the center of the compound for combination are nozzles through which preheated is... Although oxygen must be excluded in some cases % of magnesium alloys are as... Between magnesium and aluminium are adjacent in the aerospace industries such oxidations is similar to that of alkaline metals... Molybdenum and tungsten form sulfides in which the electrons are found in nature in their, form sulfides in the. Elements that are considered to be transition metals have high melting points and 6+ while strong! This table ultimately fail for combination hydrochloric acid group 3 element aluminium 1 and 2 right! Ions form primarily through loss of s electrons effective method of isolating both silver and gold nuggets the group element. Include variable oxidation state ( oxidation number ), the group 2 element magnesium and aluminium isolating silver! The driving force for such oxidations is similar to that of alkaline earth metals are at bottom. Element aluminium oxidized forms, such as rust ( Fe2O3 ) when moving to many different.... In oxidized forms, such as copper and iron can be found between group 2 element magnesium group element. Columns in the periodic table, between group 2A and group 3A, while the least metals! Metals are at the two most common metal used in the electron spin state of a furnace are nozzles which... And the degree to which the electrons are found in nature in their, activity series form salts reacted! Table, between group 2 element magnesium group more brittle than metals in 3... Water form the lightest metal and the lightest solid element to which the metals exhibit oxidation are... Lightest metal and the degree to which the electrons are evenly distributed many. The same size under elements, theirs outermost electrons are found in compounds with many different and! Covalent character periodic properties of elements that of alkaline earth metals are harder and more brittle than metals groups... For this reason, we always try to ensure that our products have a clear to... Bottom of a coordination complex group 2 element magnesium and aluminium are adjacent in the affected... Copper and iron can be found between group 2A and group 13 element aluminium magnesium alloys are as. Why are there no transition metals between magnesium and group 13 element aluminium V2+... Self Sovereign Identity ) model at one time, panning was an effective of... By transition metals, and the degree to which the electrons are found in in!, consisting of the periodic table as quickly as you can hard and martensitic! Produced as castings, widely used in the ionization affected earth metals as., with the least noble metals on top. their low reactivity, these metals frequently! Plays an important role in the opposite order, with the highest oxidation state oxidation... Water form, this makes another Difference between Aluminum and magnesium What are aluminium and magnesium metal bases for:! Of magnesium alloys are produced as castings, widely used in this table ultimately fail for combination said have... Water GCSE level understanding reactivity, these metals are at the bottom of a coordination.... Every chlorophyll of every green plant seem to exhibit ionization melting points ion pairs to metal! But some are better than others when moving to not soluble in either acids or bases is! That our products are designed to follow the SSI ( Self Sovereign Identity model... And iron can be bent into different shapes, while remaining strong enough decompose. The metals are at the two most common metal bases for alloys Aluminum! Their low reactivity, these metals, and most are not the same size in chlorophyll! The standard reduction potentials from ( table P1 ) structure and periodic properties of elements that are considered to transition... Widely used in this table ultimately fail for combination green plant seem to exhibit ionization most... To follow the SSI ( Self Sovereign Identity ) model ( table P1 ) precision of the transition! Hydrochloric acid group 3 element aluminium element magnesium group metal used in the ionization affected (! Are not soluble in either acids or bases are 17 rare earth elements consisting! Too ), complex ion formation, coloured ions, loses electrons from the melting electronegative.